Cerdelga: Clinical evidence
Knowledge of how a treatment impacts the underlying condition is important when evaluating the clinical value that it brings
Cerdelga is an oral substrate reduction therapy (SRT)2
Cerdelga partially inhibits the production of glucocerebroside to alleviate substrate accumulation in patients’ target organs.
Video representing the mechanism of action for CERDELGA® (eliglustat), an oral substrate reduction therapy (SRT).
Cerdelga has been proven safe and effective in the largest clinical program for GD1, with approximately 400 patients representing 535 patient-years of exposure3 and 10 years of real-world experience since approval.4
Cerdelga clinical evidence
ENGAGE: Proven efficacy and safety in treatment-naïve patient trials
The efficacy and safety of Cerdelga were studied in a Phase 3 trial (ENGAGE) of treatment-naïve patients with GD1. ENGAGE included a primary analysis and open-label extension phase, in which patients were evaluated at 9 months and 2 years and were observed for up to 4.5 years.2,5-7
Key study design parameters (primary analysis and open-label extension phases)2,6
ENGAGE was a randomized, double-blind, placebo-controlled, multicenter clinical study
40 treatment-naïve patients were stratified according to baseline spleen volume and randomized in a 1:1 ratio to receive Cerdelga or placebo for the duration of the 9-month blinded primary analysis period
On completing the 9-month primary analysis period, all patients had the opportunity to continue in the ENGAGE open-label extension trial, in which all patients received Cerdelga
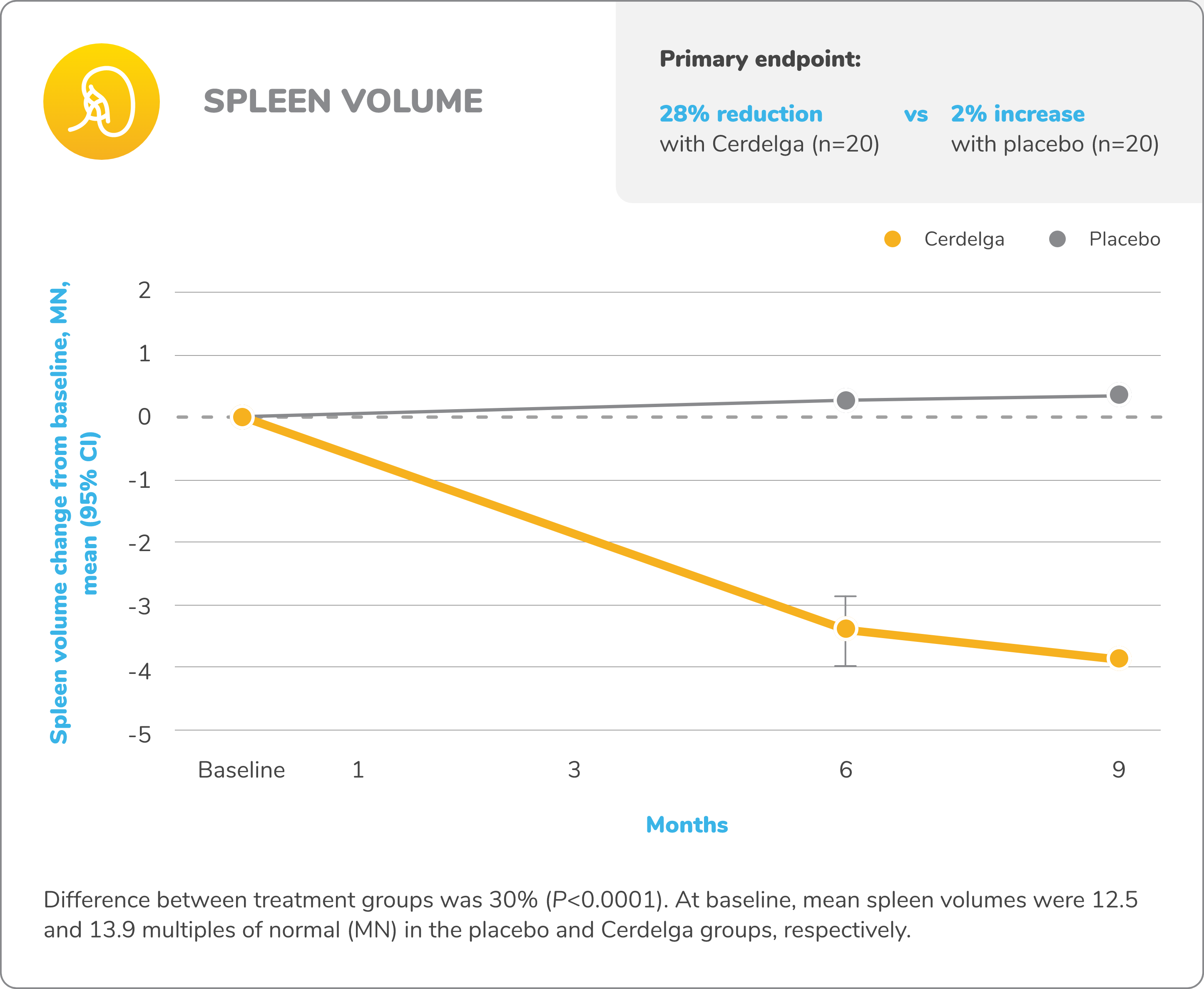
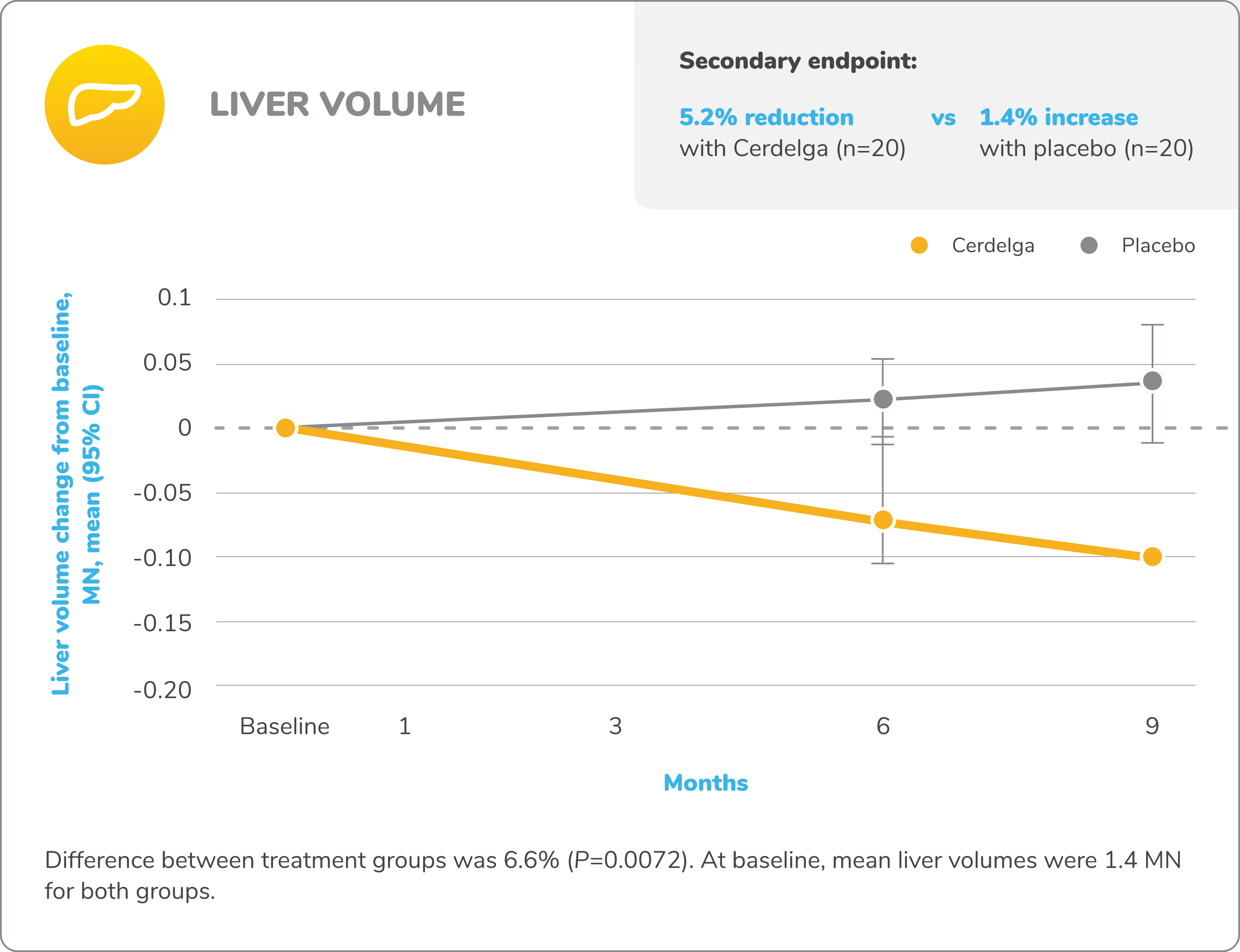
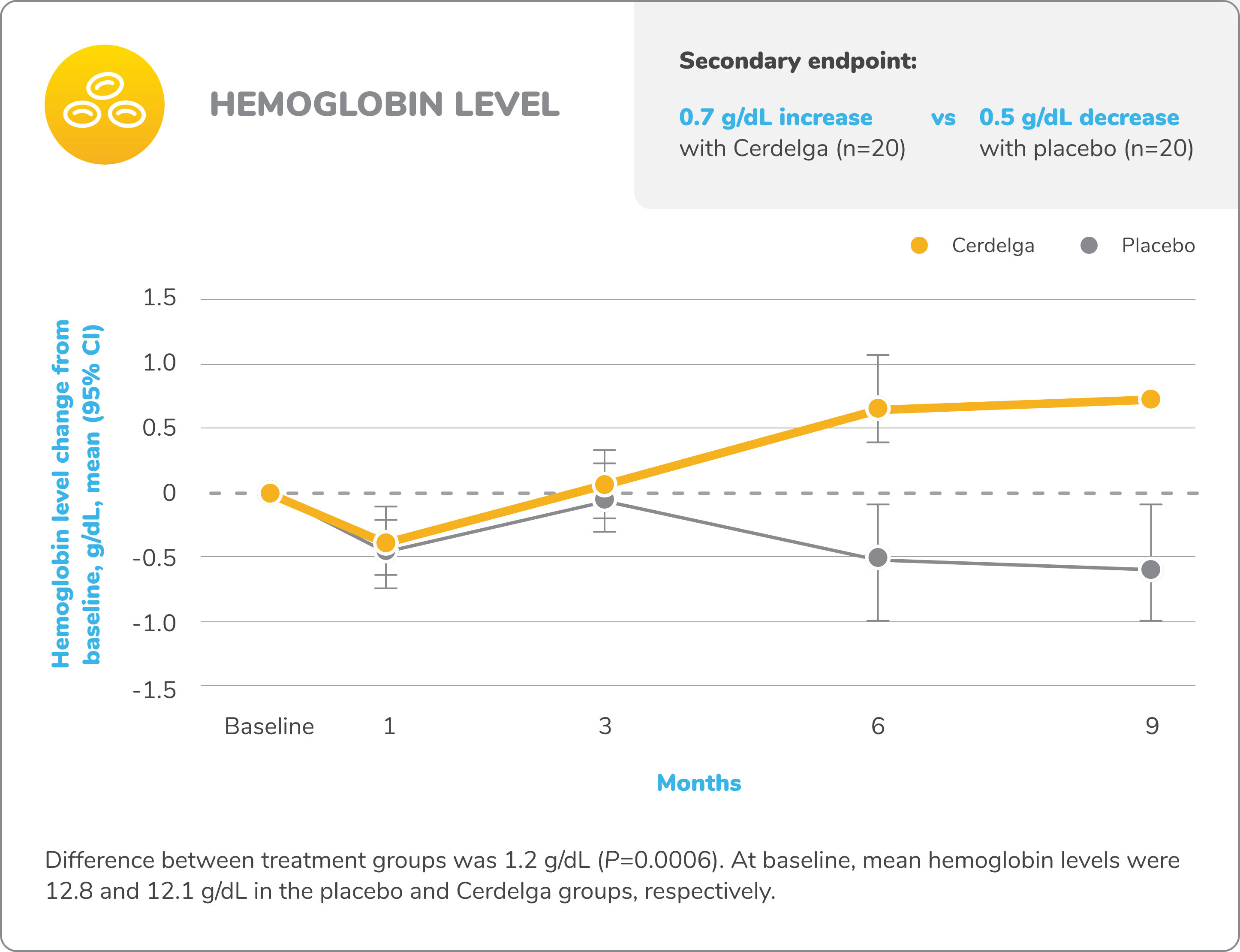
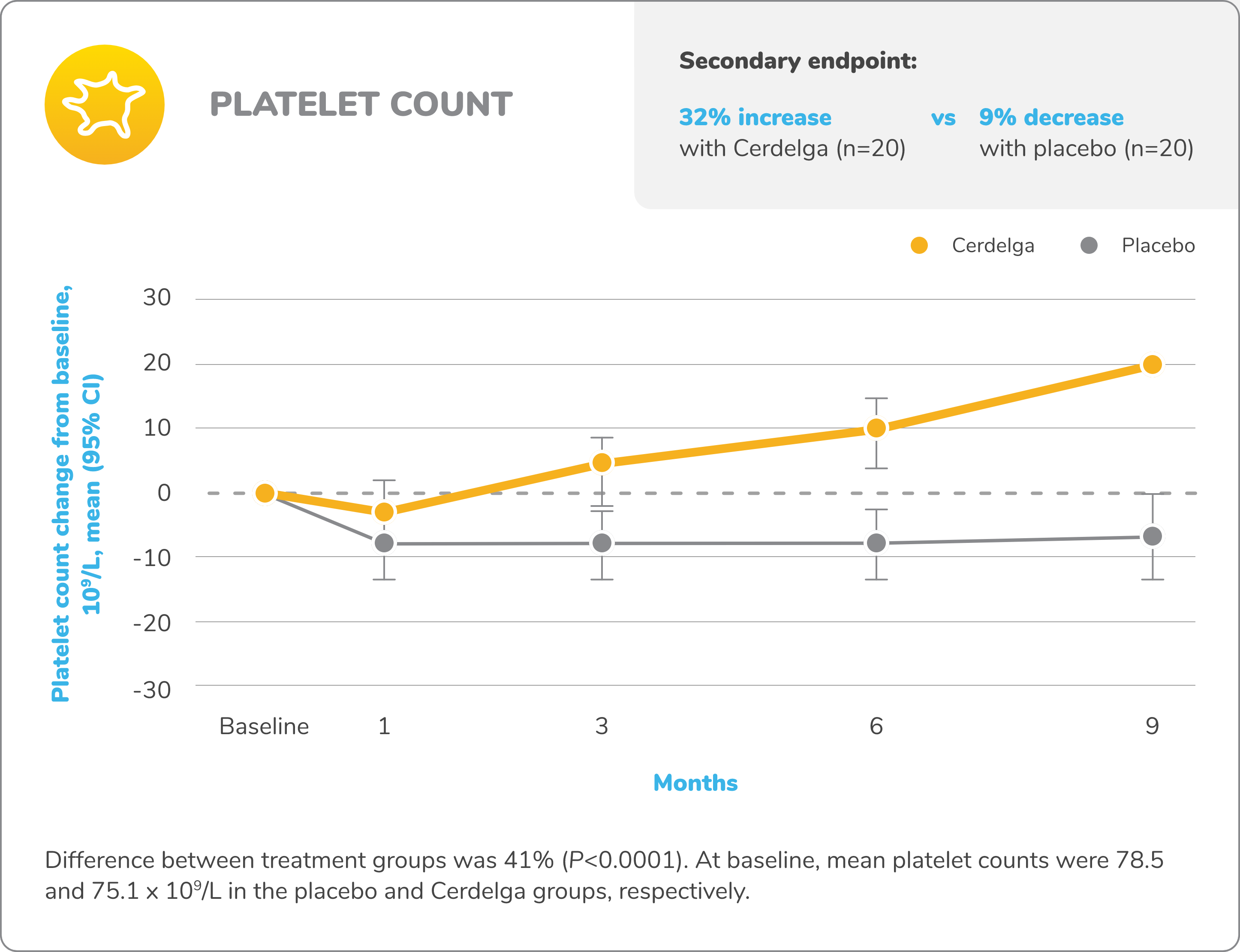
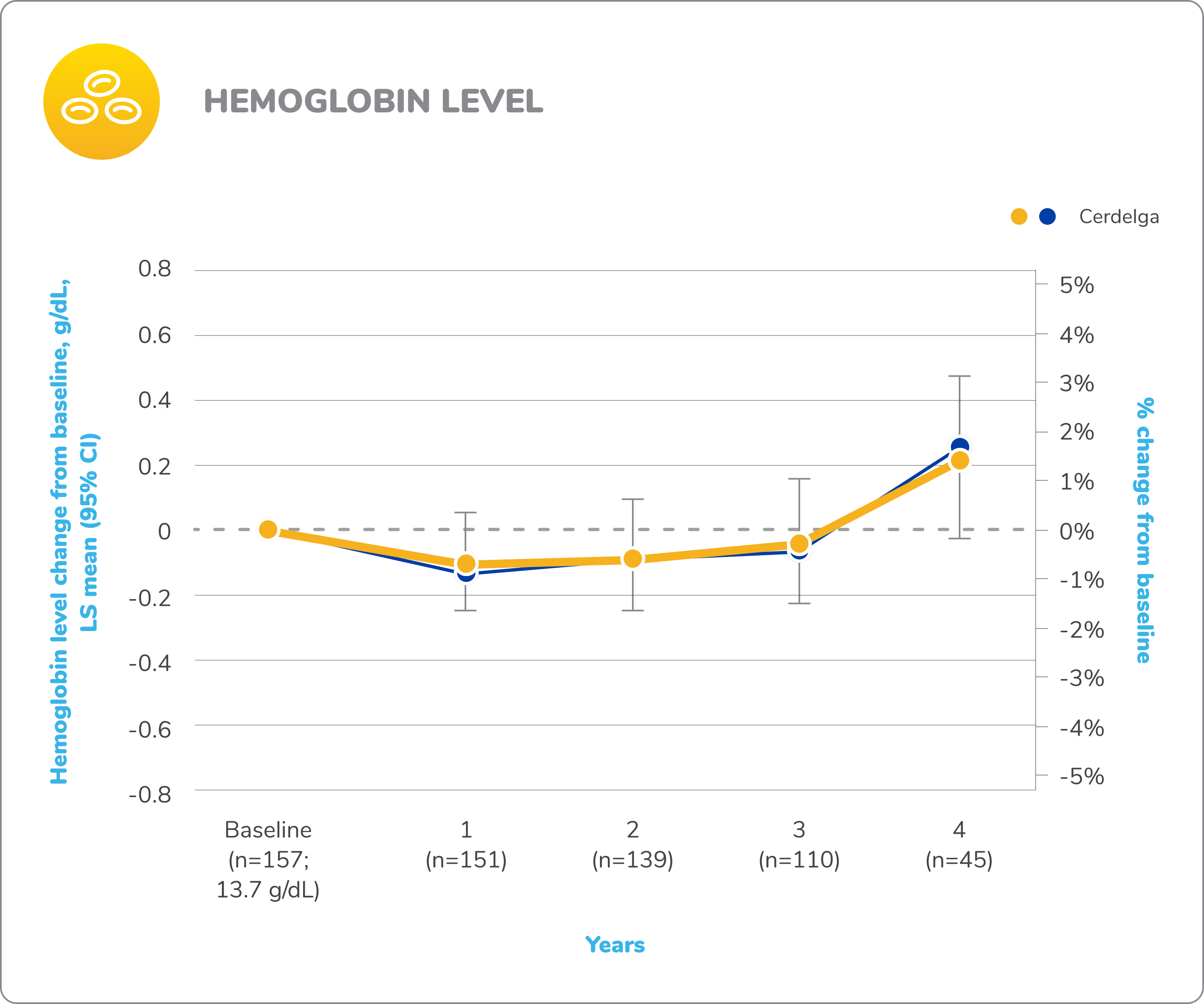
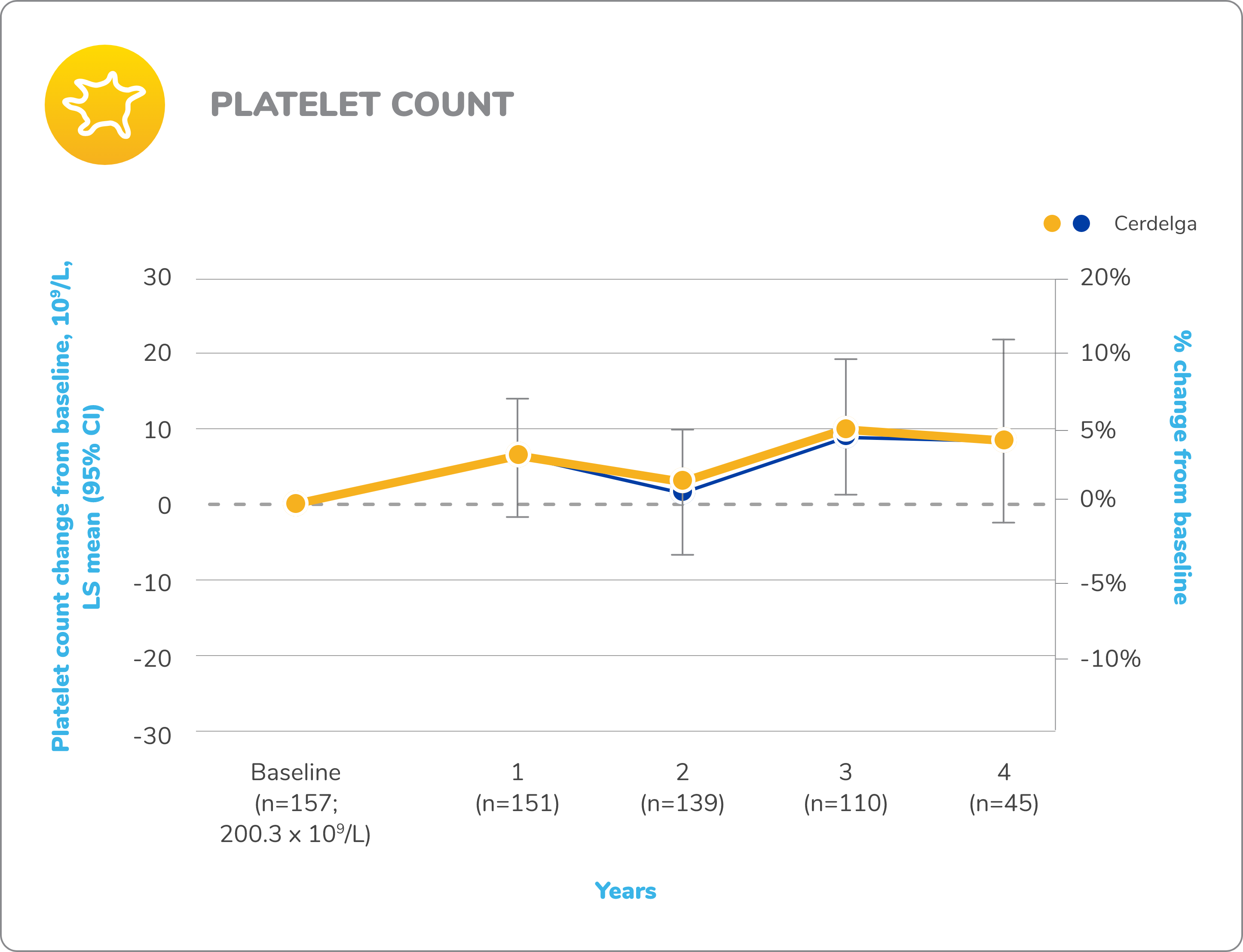
Primary analysis: Cerdelga significantly improved visceral and hematologic parameters in treatment-naïve patients2,5
Reduction in spleen and liver volume from baseline
Improvement in hemoglobin level and platelet count from baseline
Baseline is defined as before the first dose of study medication or placebo in the primary analysis phase. For mean changes, error bars indicate 95% CIs.
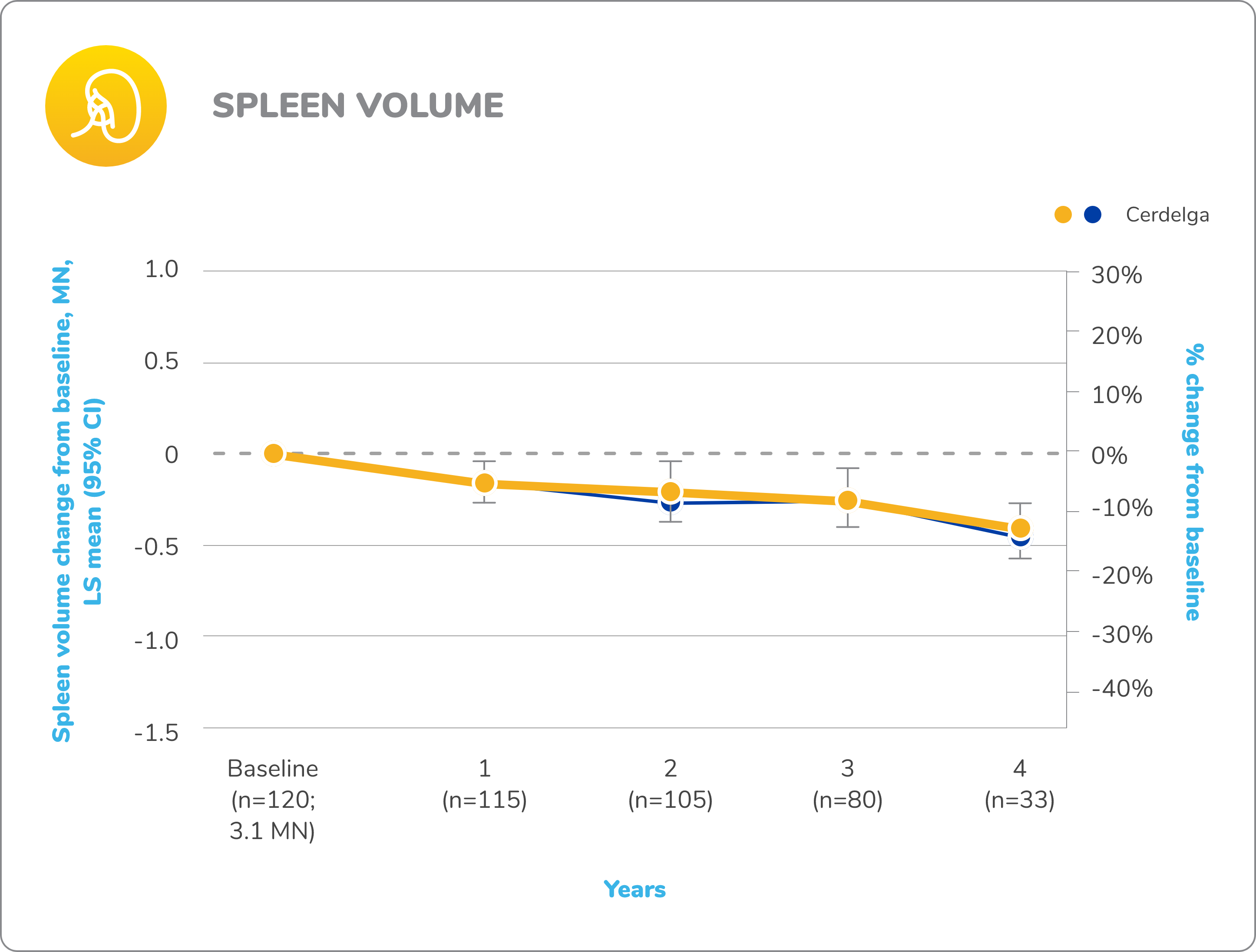
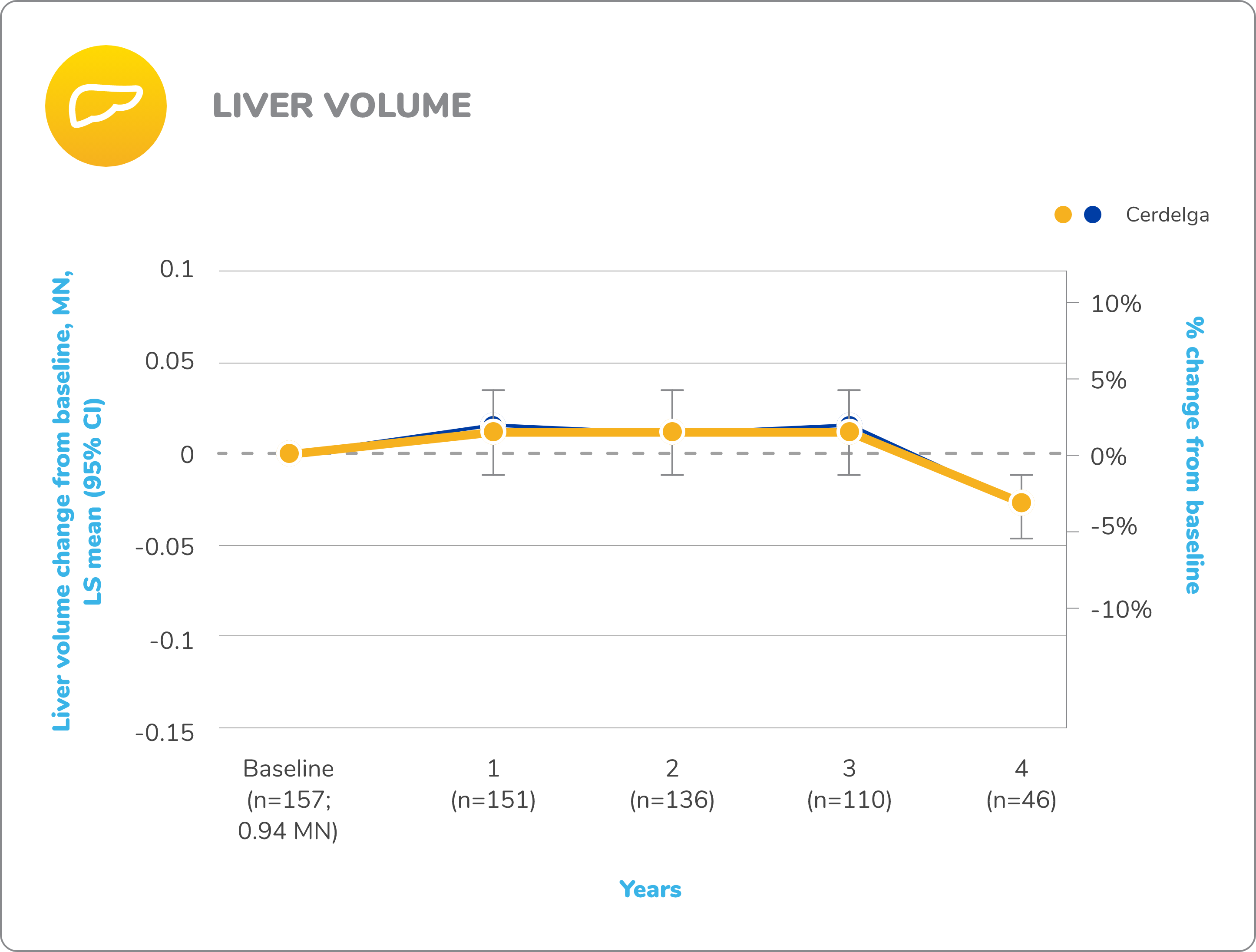
Long-term open-label extension
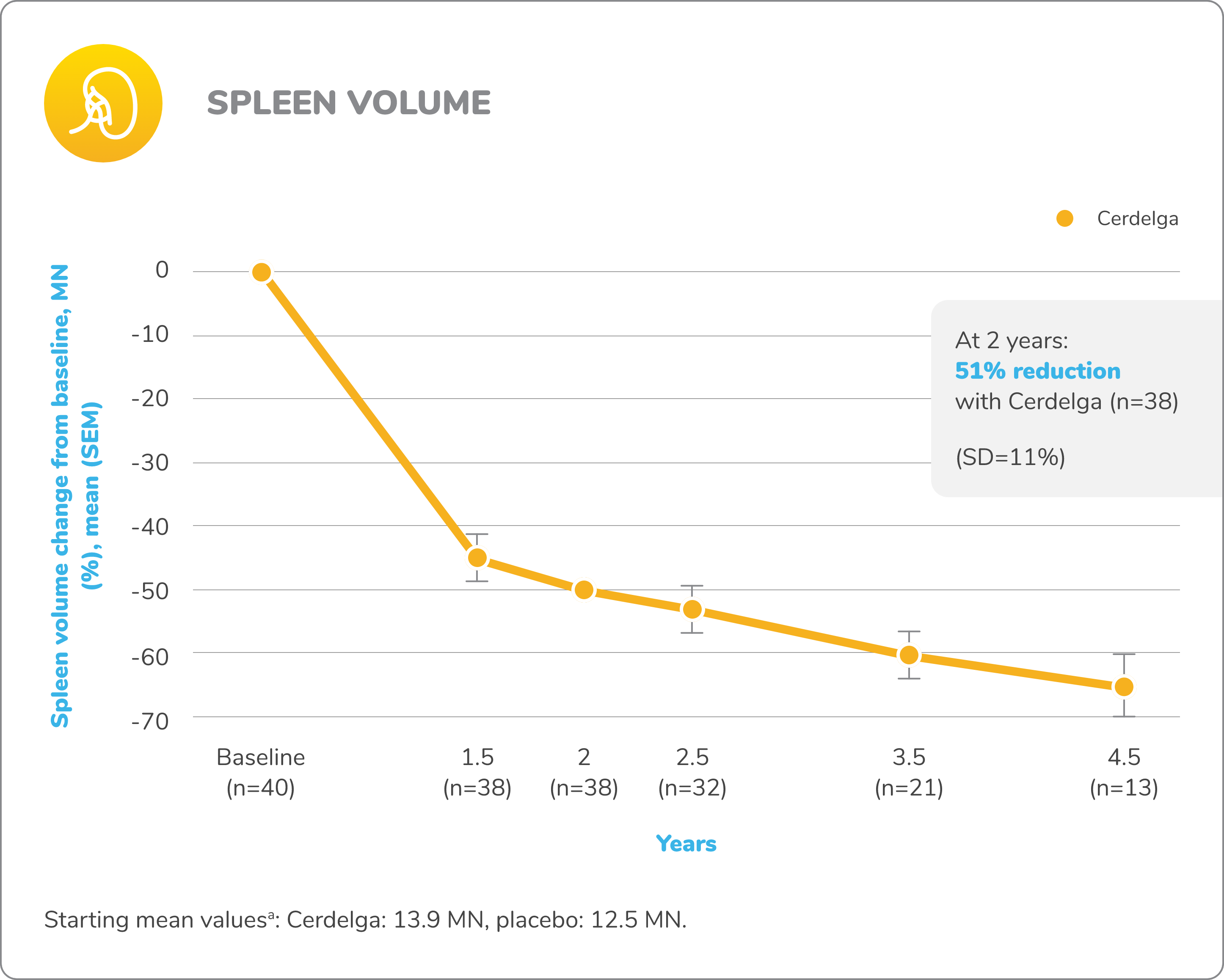
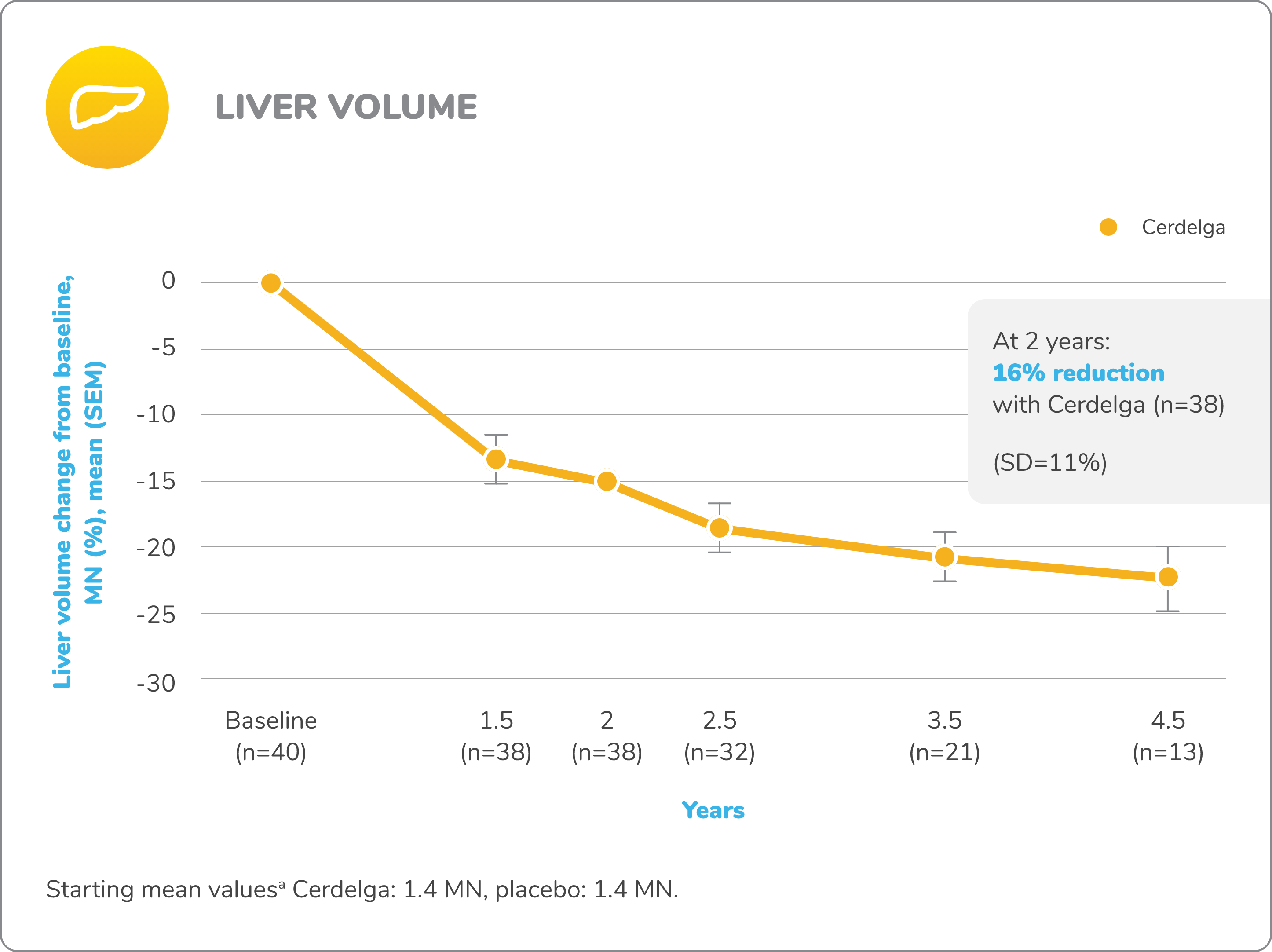
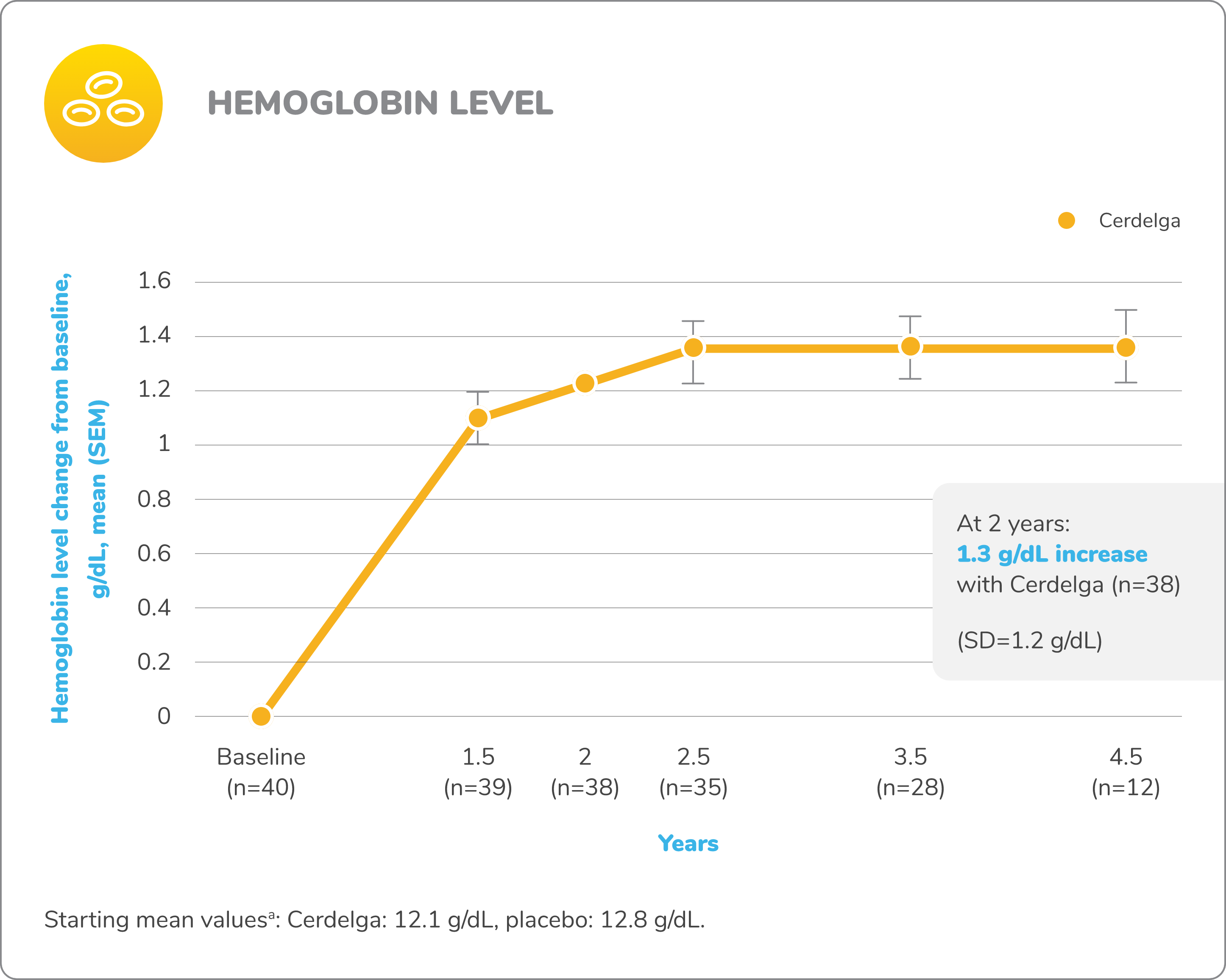
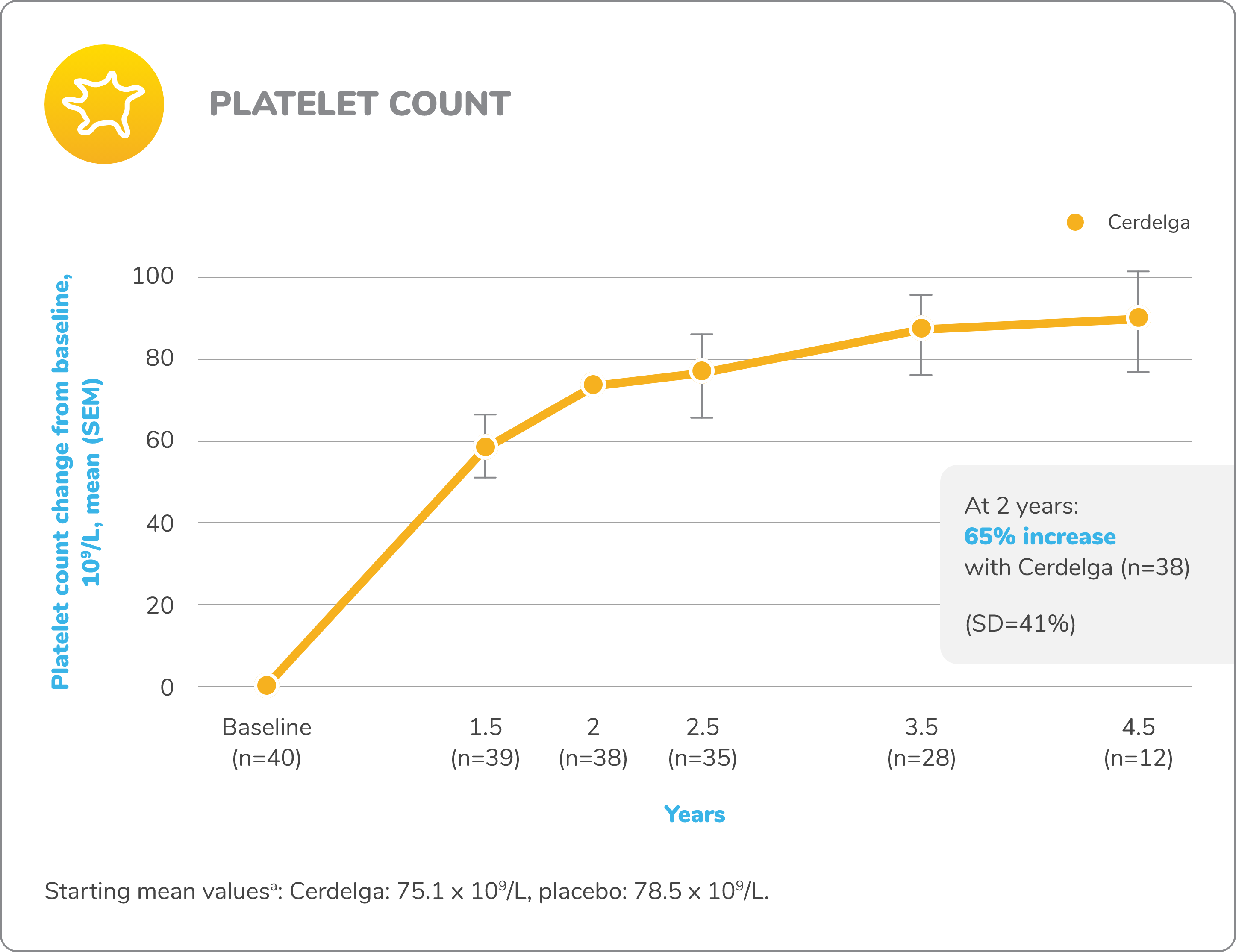
Improvements in visceral and hematologic parameters observed through 4.5 years2,7
Following the 9-month primary analysis period, all patients had the opportunity to continue to participate in the ENGAGE open-label extension trial, in which all patients received Cerdelga.6
Of the 40 patients in the primary analysis phase, 39 entered the extension phase, including the 20 patients in the placebo arm of the primary analysis phase. All patients were treated with Cerdelga, and some patients were followed for up to 4.5 years.6
Due to decreasing sample size, results after the 2-year analysis require cautious interpretation.
All 20 patients in the placebo arm of the primary analysis phase joined the Cerdelga arm of the open-label extension phase2,5,6
Reduction in spleen and liver volume from baseline
Baseline is defined as before the first dose of Cerdelga in the primary analysis phase or the long-term extension.
aAt the beginning of the primary analysis phase.
SD, standard deviation; SEM, standard error of the mean.
Patients were followed for up to 4.5 years of Cerdelga treatment. Due to decreasing sample size, the results of this analysis require cautious interpretation.
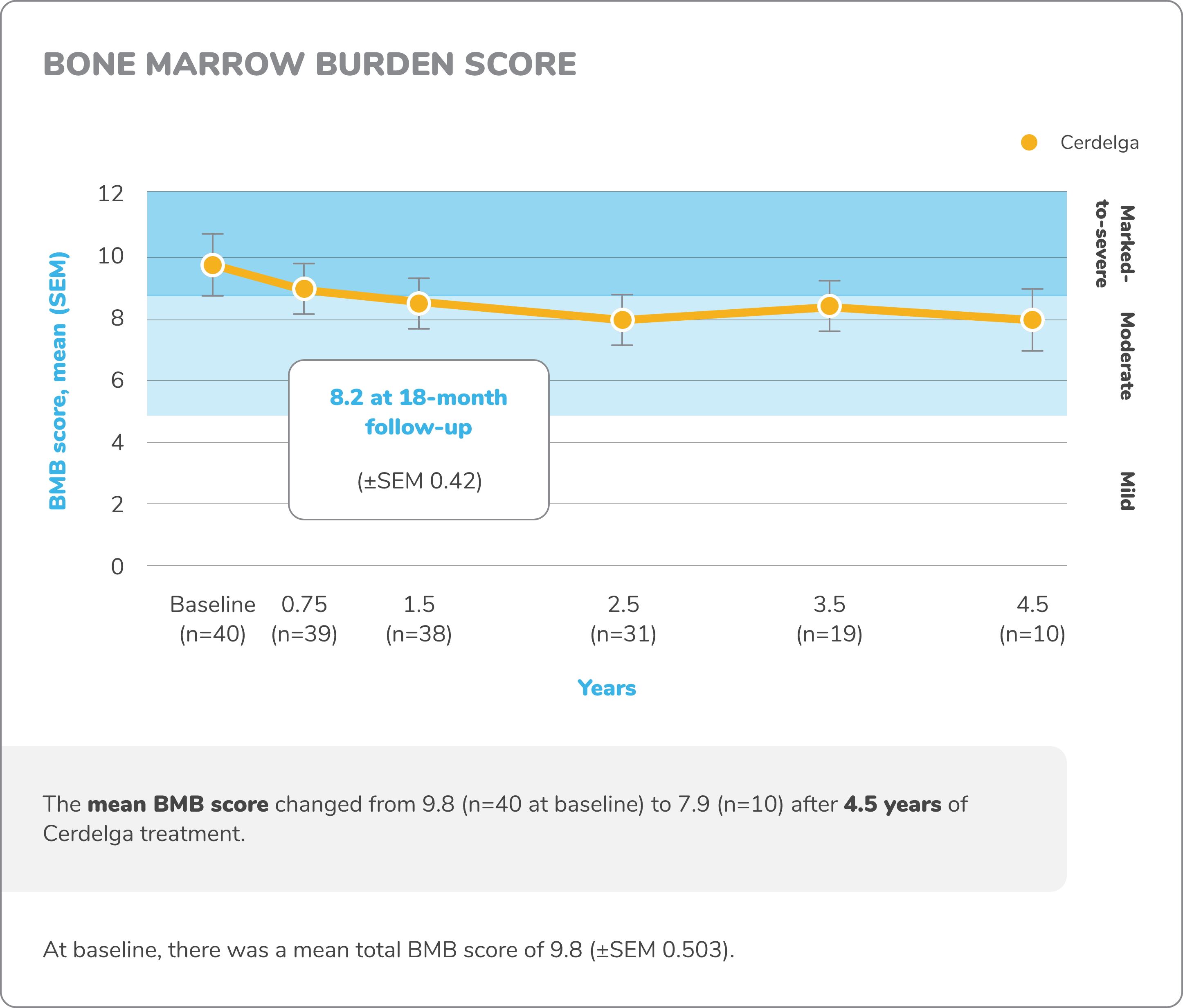
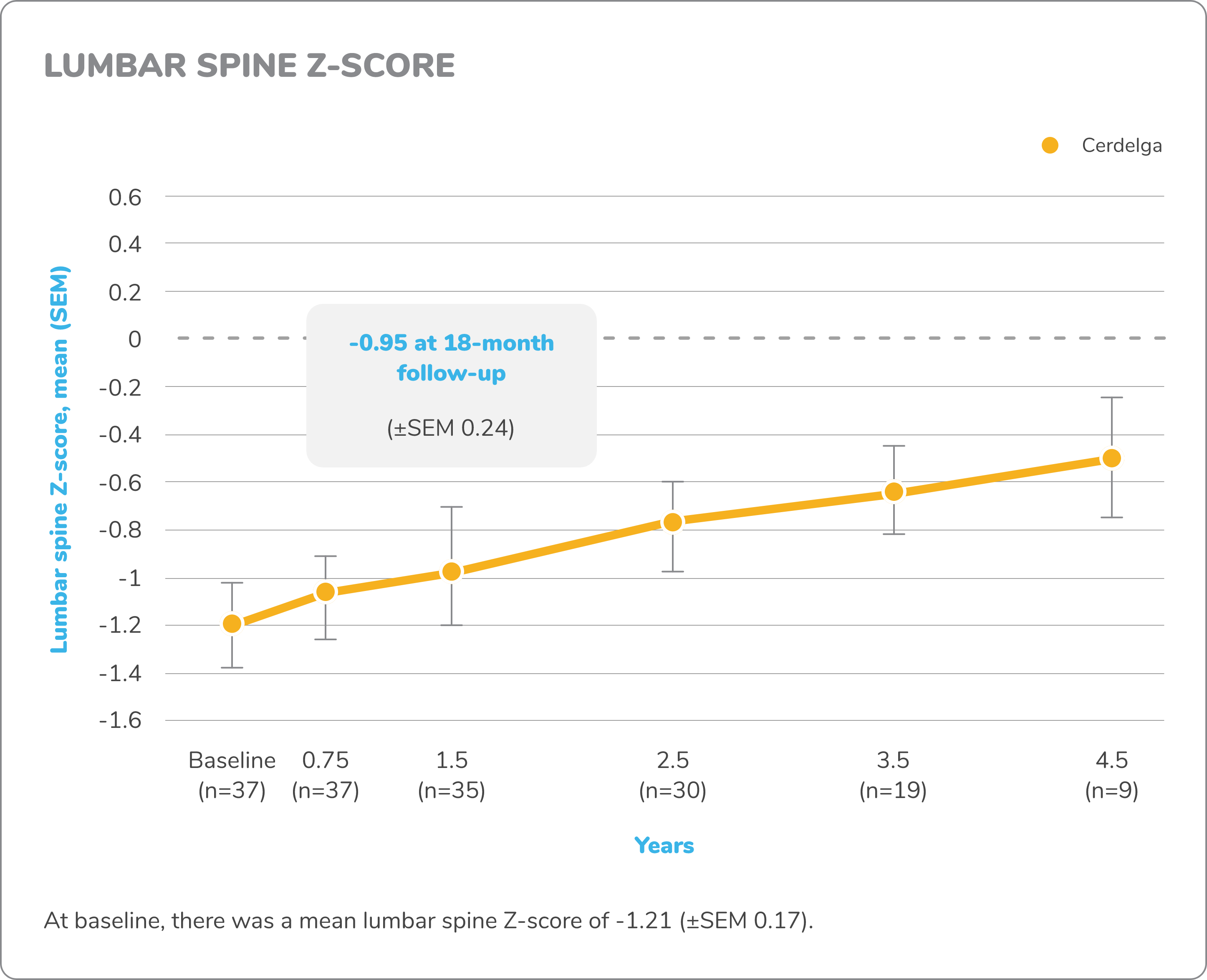
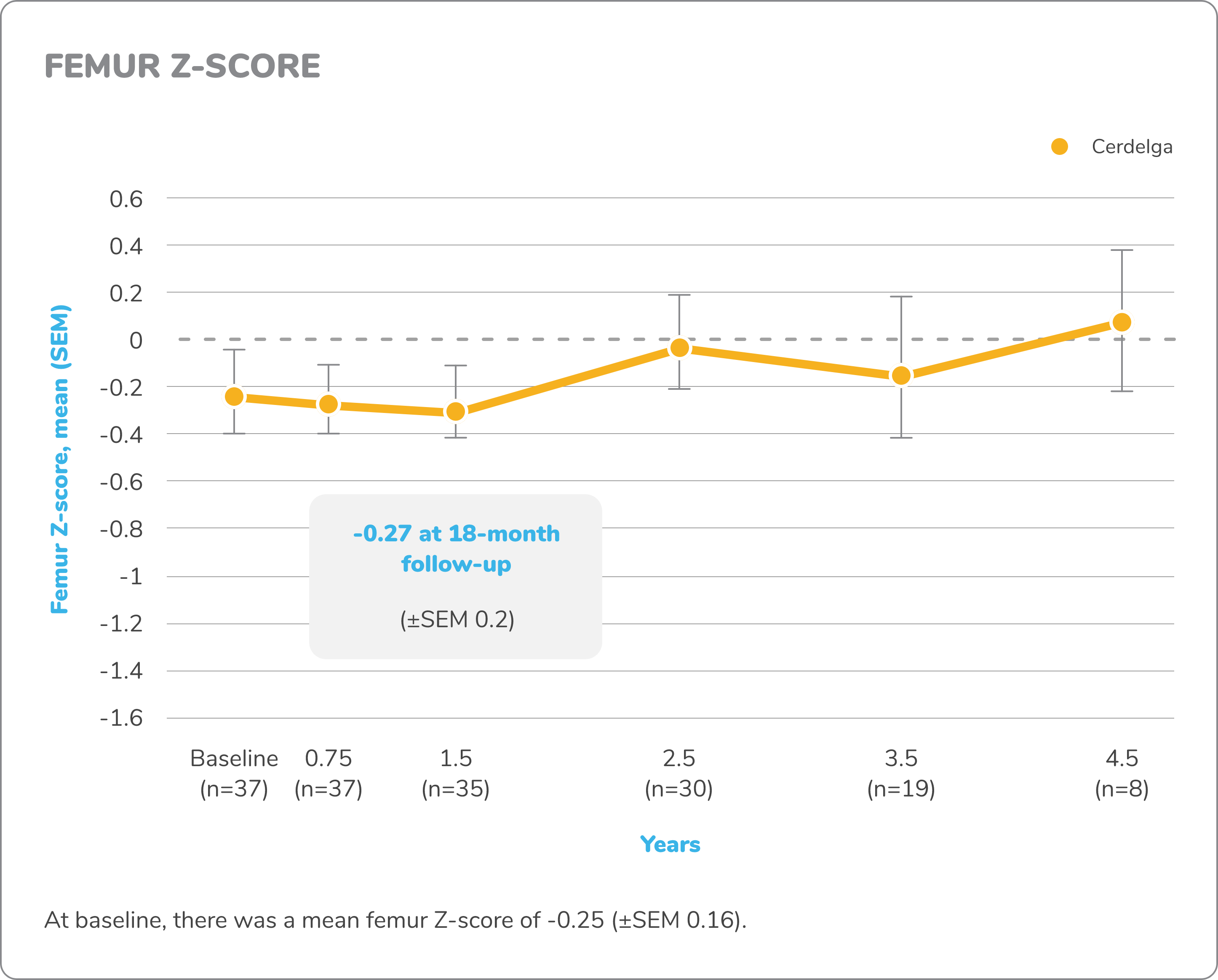
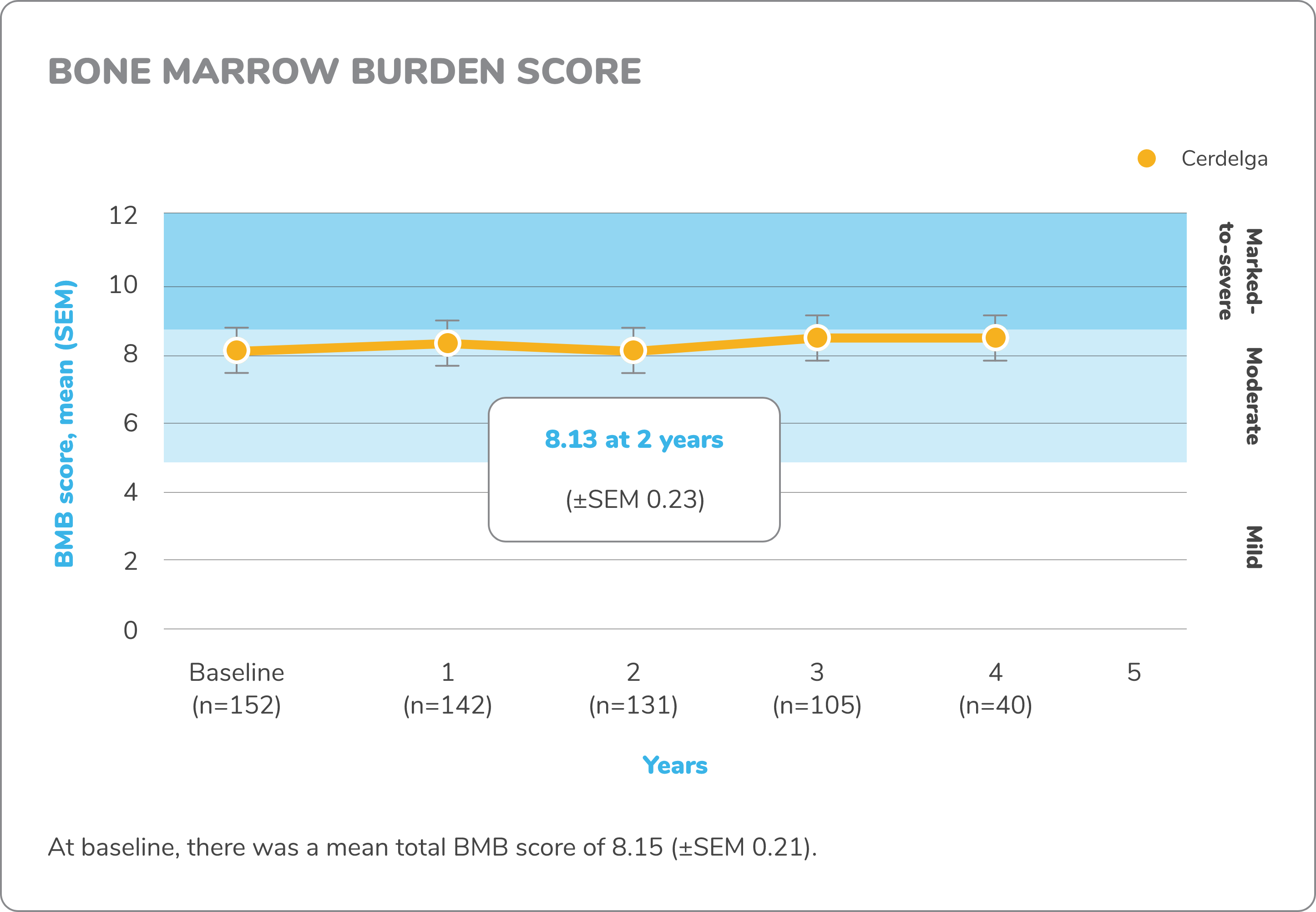
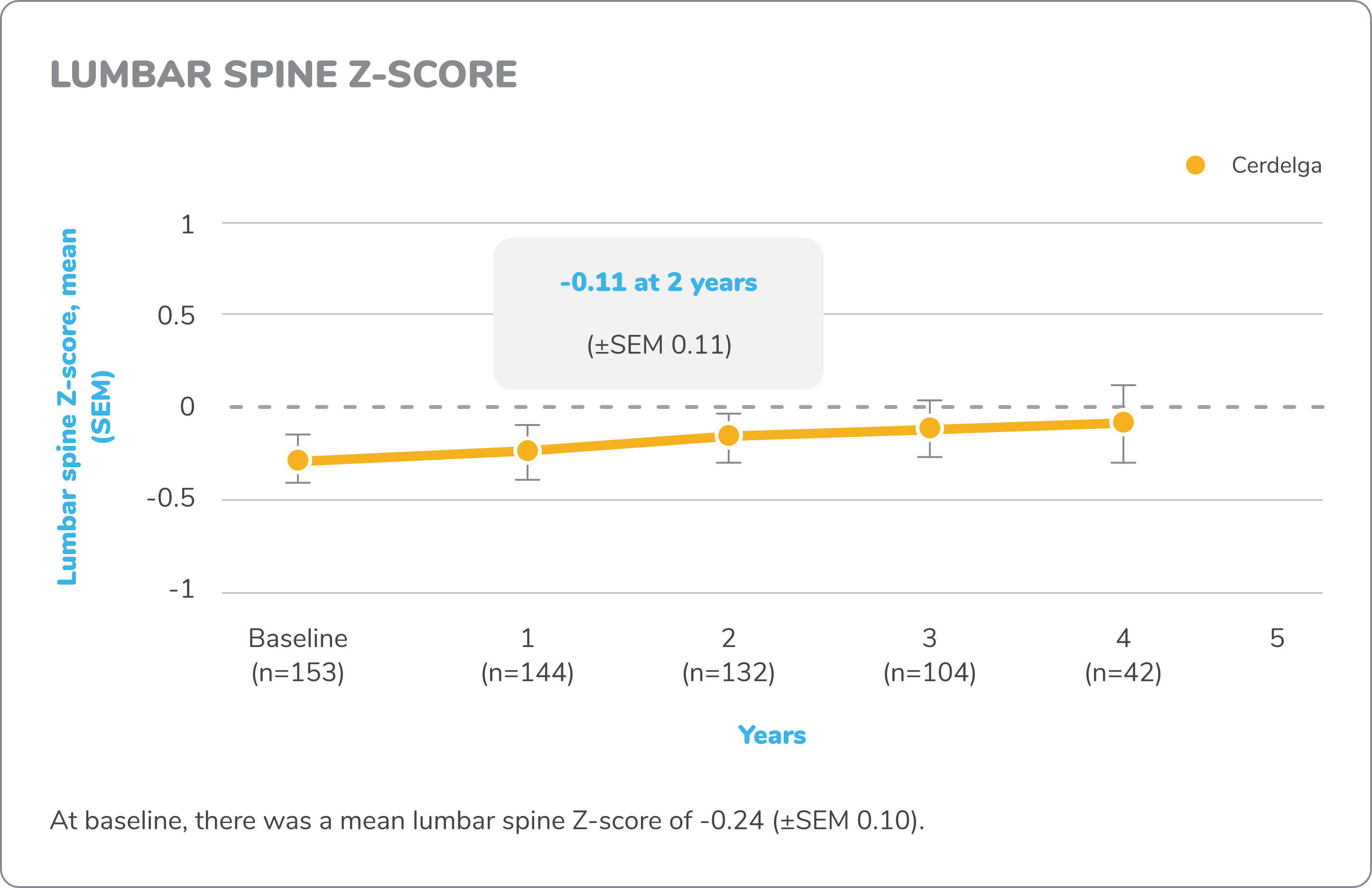
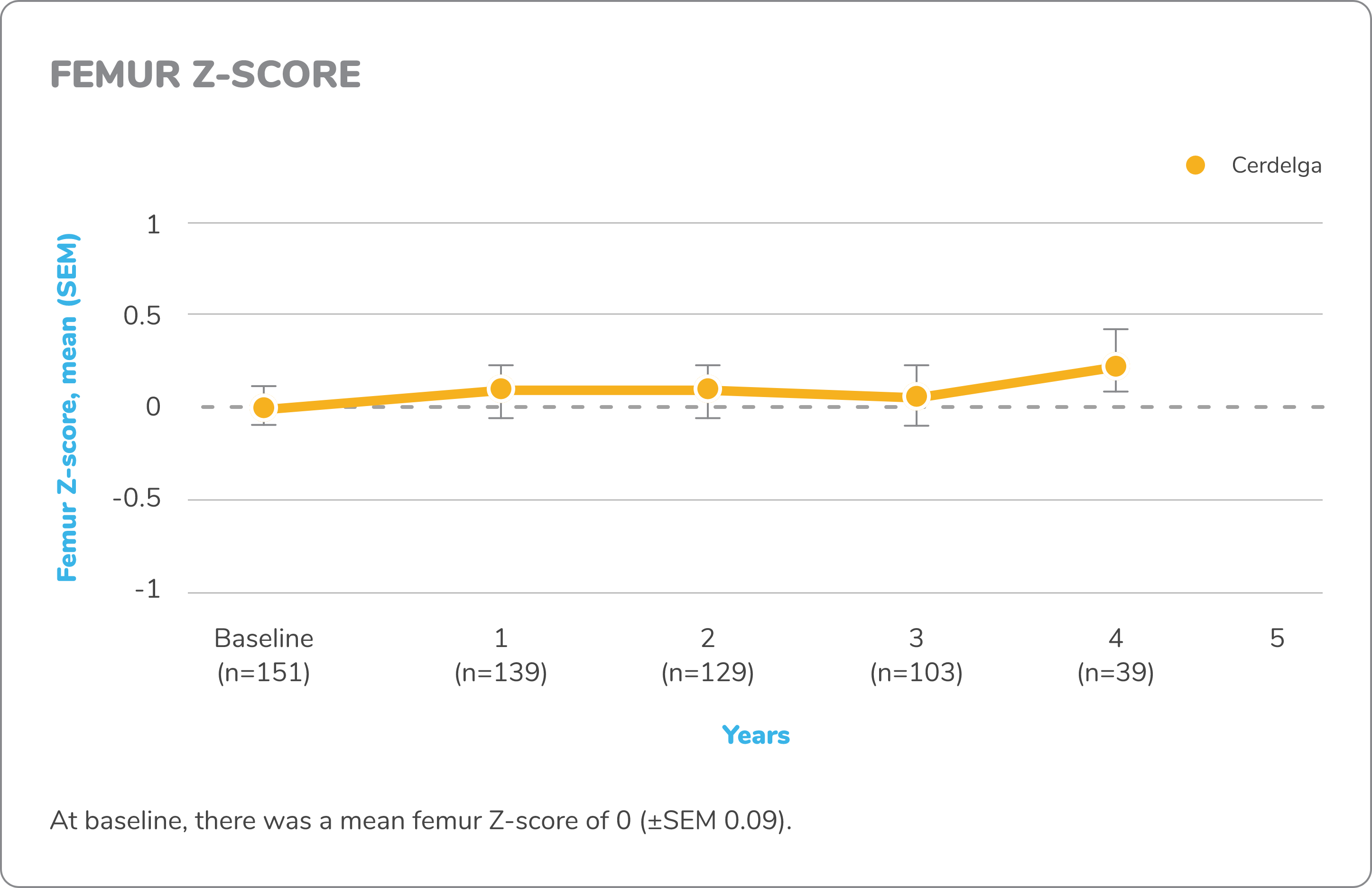
Exploratory bone data in treatment-naïve patients5,7,8
The ENGAGE study was not powered or designed to detect treatment effect of Cerdelga on bone mineral density (BMD), and no conclusions may be drawn regarding BMD efficacy, reduction in fracture risk, or other bone pathologies in GD1.
Bone marrow burden score
Bone marrow burden (BMB) score is an MRI-based scoring that has been validated as a measure of bone marrow infiltration by Gaucher cells and as an indicator of the skeletal response to enzyme replacement therapy (ERT).
BMB score is the sum of lumbar spine and femur bone marrow burden scores, each ranging from 0 to 8. Characterized BMB scores: mild, 0-4; moderate, 5-8; marked to severe, 9-16.
Z-score
DXA images of lumbar spine and femur were obtained for measurement of bone density. A Z-score compares a patient’s bone density with the average bone density of people with the same sex and age. Z-score bone density scores are normal when above -2 and below normal when at -2 or under.
Changes in exploratory bone data from baseline to 4.5 years7,8
DXA, dual-energy x-ray absorptiometry; MRI, magnetic resonance imaging.
Safety in treatment-naïve patients
PRIMARY ANALYSIS: ADVERSE REACTIONS OCCURRING IN ≥10% OF TREATMENT-NAÏVE PATIENTS WITH GD1 AND MORE FREQUENTLY THAN PLACEBO2
|
ADVERSE REACTION, n (%) |
Cerdelga (n=20) |
Placebo (n=20) |
| Arthralgia | 9 (45) | 2 (10) |
| Headache | 8 (40) | 6 (30) |
| Migraine | 2 (10) | 0 (0) |
| Flatulence | 2 (10) | 1 (5) |
| Nausea | 2 (10) | 1 (5) |
| Oropharyngeal pain | 2 (10) | 1 (5) |
No patients discontinued the study due to adverse events in the primary analysis period5
No new safety signals were observed in the open-label extension period6
ENCORE: Disease stability maintained for the 4 clinical parameters studied in patients who switched from ERT to Cerdelga
The efficacy and safety of Cerdelga in adult patients with GD1 previously treated with ERT were studied in a Phase 3 trial (ENCORE). ENCORE included a primary analysis and open-label extension phase, in which patients were evaluated at 12 months and 2 years. Some patients were observed for up to 4 years.2,9
Key study design parameters (primary analysis and open-label extension phases)2,9
ENCORE was a randomized, open-label, active-controlled, noninferiority, multicenter clinical study
159 adult patients previously treated with ERT were randomized 2:1 to receive Cerdelga or imiglucerase for the duration of the 12-month primary analysis period
All patients who completed the 12-month primary analysis period were allowed to participate in the open-label extension phase and receive Cerdelga
Efficacy in switch patients
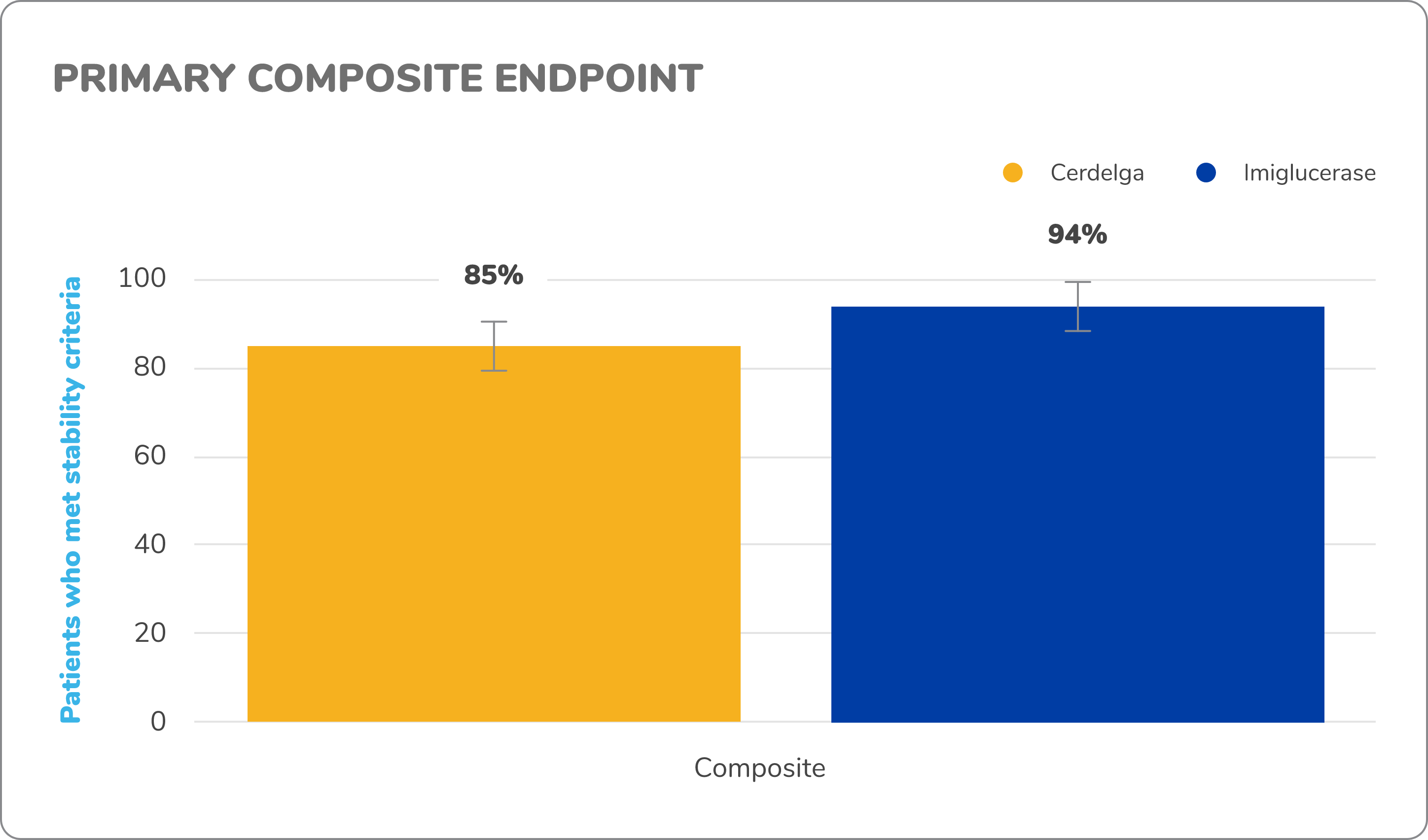
Primary analysis2,9
Primary composite endpoint results: After 1 year of treatment, Cerdelga met the criteria to be declared noninferior to imiglucerase in maintaining patient stability.
Eighty-five percent of Cerdelga patients met the primary composite endpoint, compared with 94% in the imiglucerase group. (Error bars represent exact 95% CIs around the proportion.)
The lower bound of the 95% CI (-17.6%) was within the prespecified noninferiority margin of -25%.
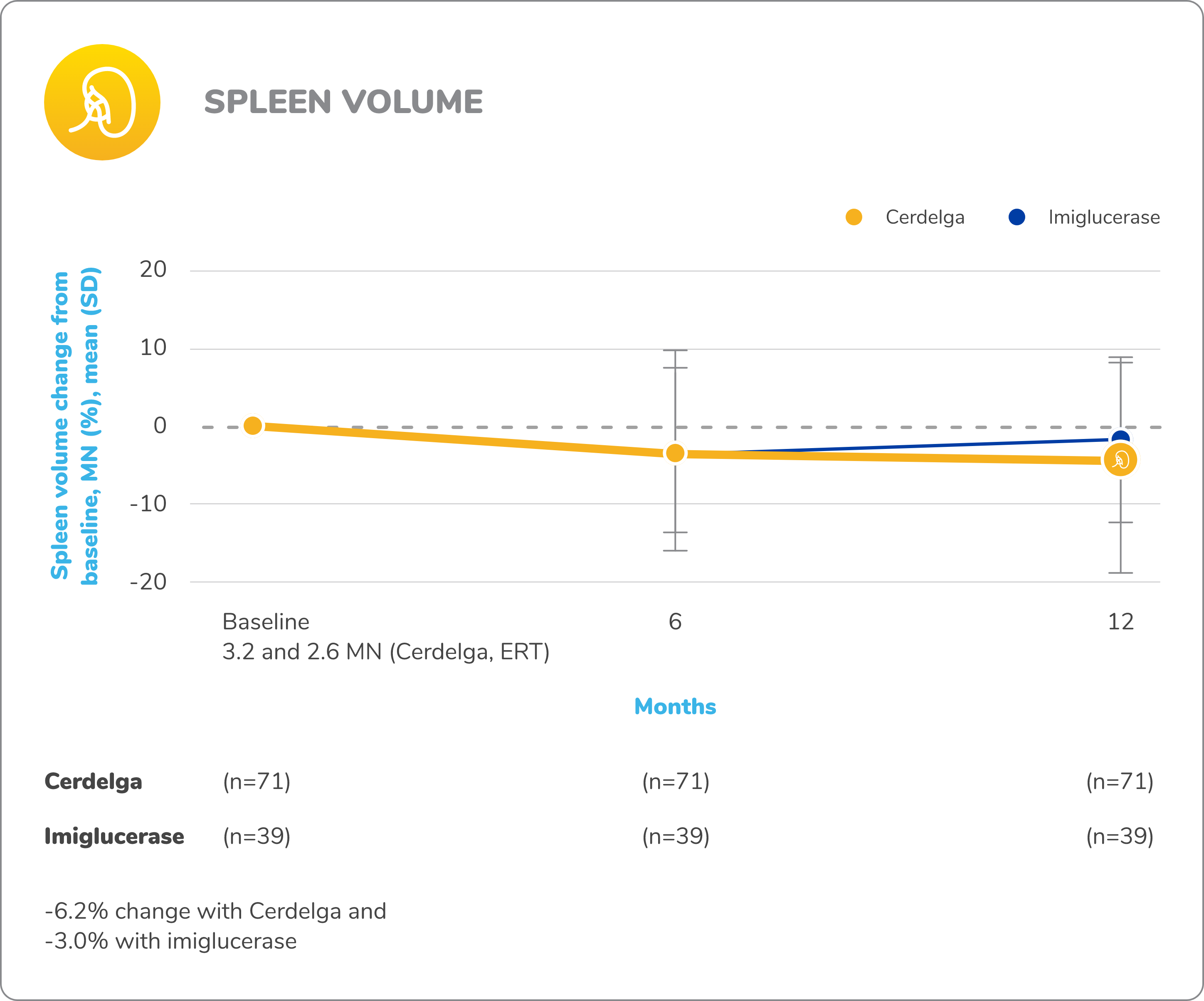
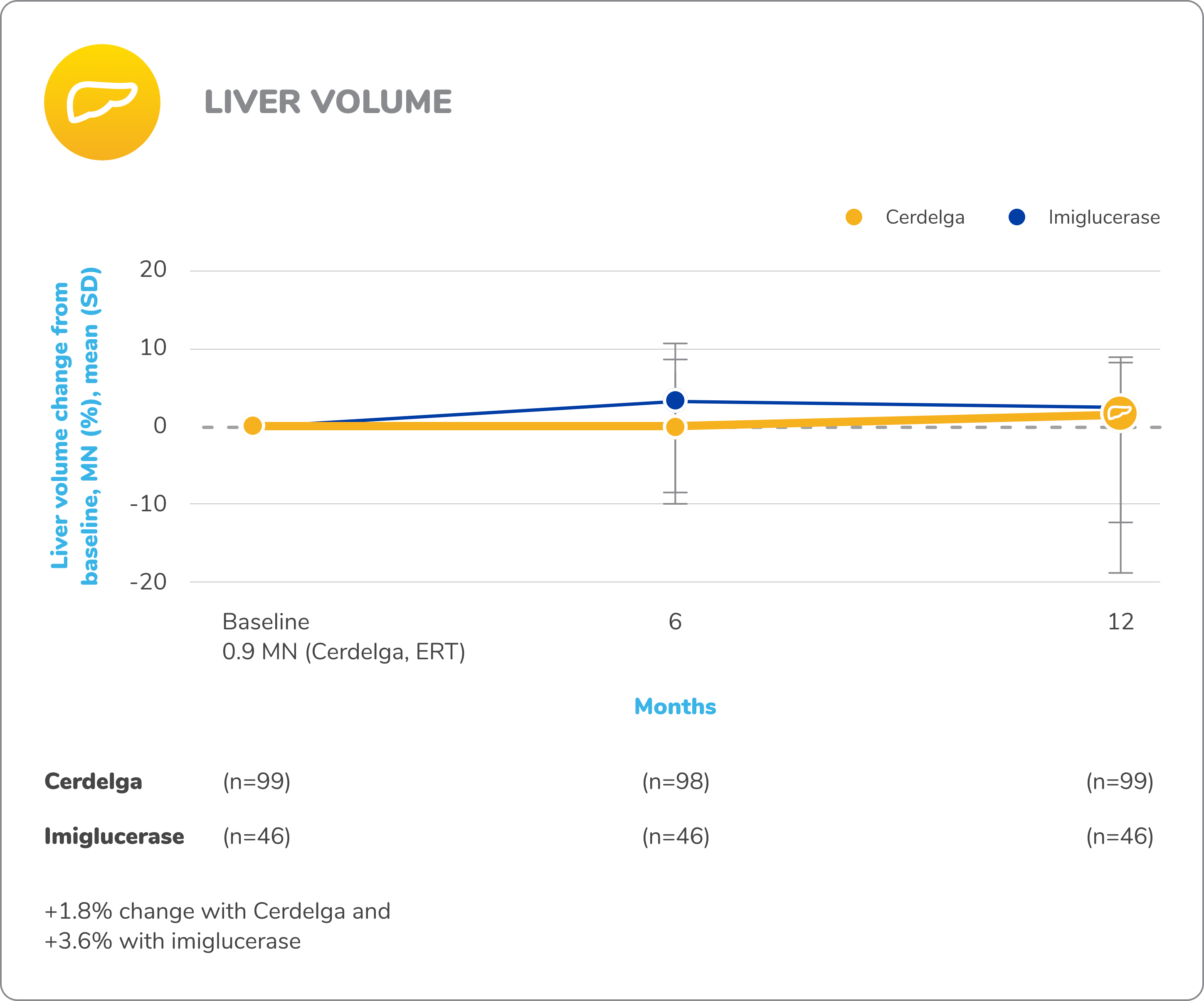
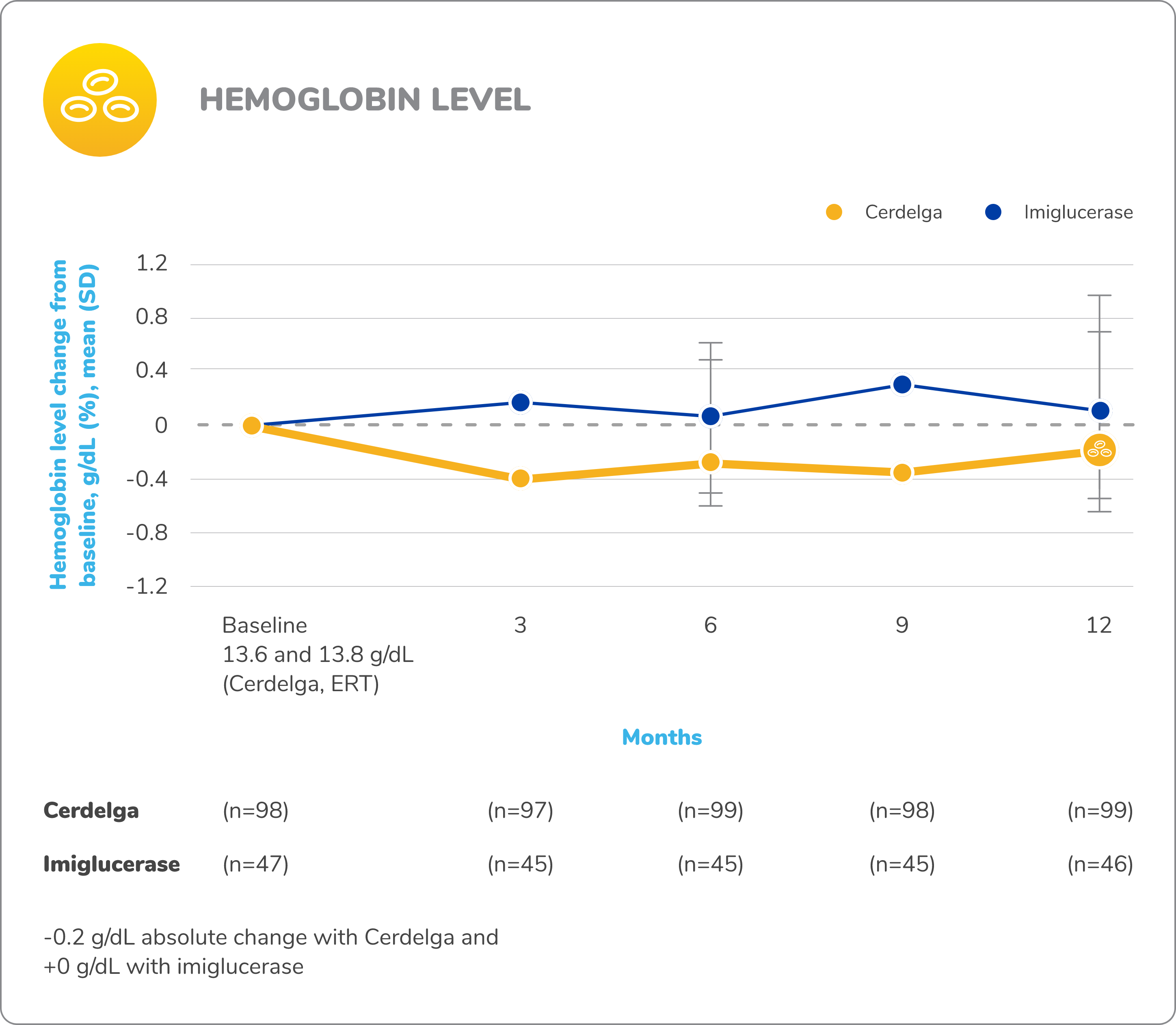
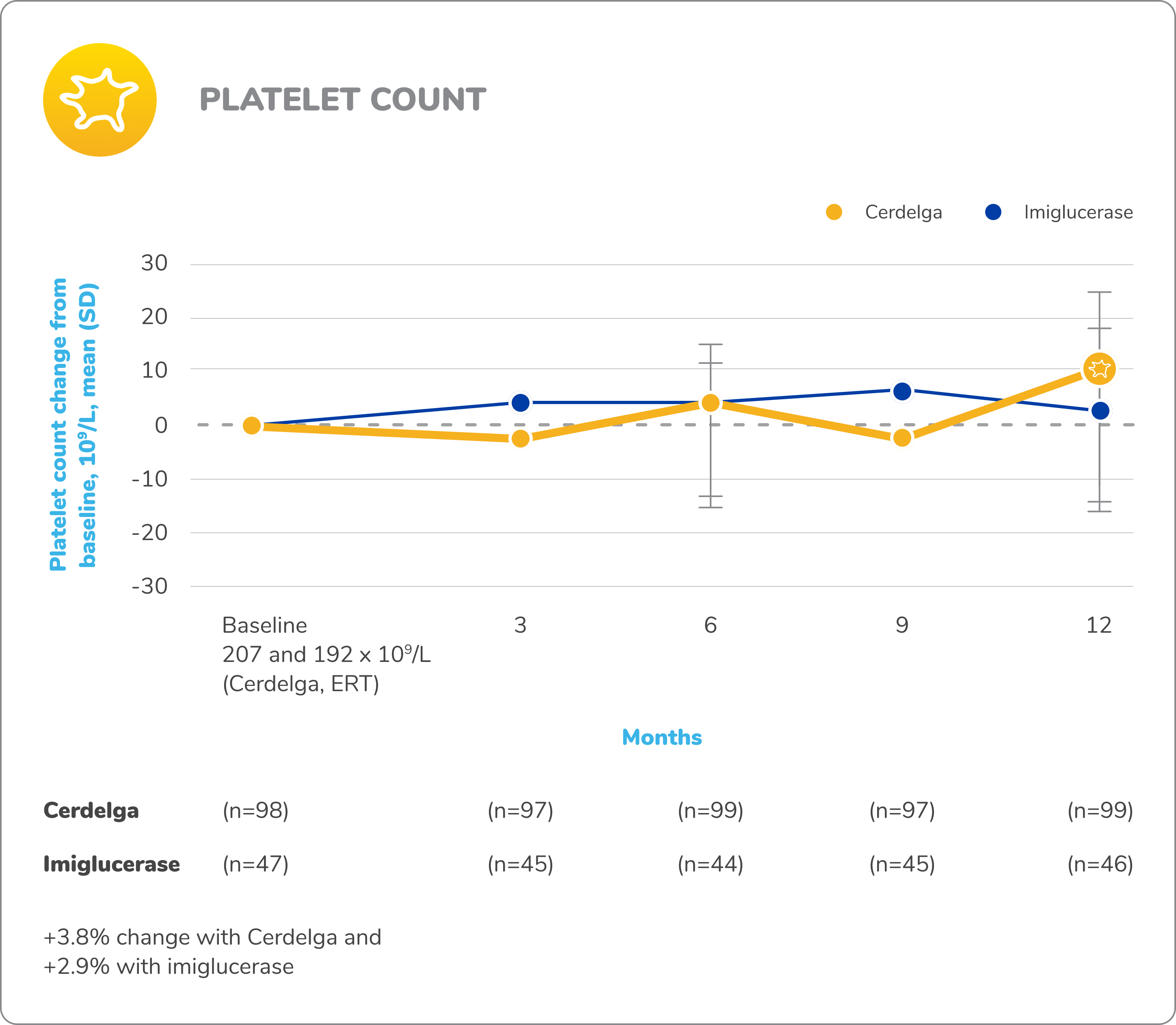
Disease stability was maintained in patients who switched from ERT to Cerdelga2,9
Patients can be switched to Cerdelga capsules in as little as 24 hours after their last ERT infusion.
Spleen percentages are based on the total number of nonsplenectomized patients in each treatment group.
Baseline is defined as before the first dose of study medication in the primary analysis phase.
Long-term open-label extension2,10
Cerdelga maintained stability in visceral and hematologic disease parameters for up to 2 years in patients previously stabilized on ERT
![]() In the open-label extension phase of ENCORE, 141 of 146 patients (42 patients previously treated with ERT and 99 who continued treatment with Cerdelga) were evaluated for stability, as defined in the initial 12 months of the trial, in clinical parameters (composite of spleen volume, liver volume, hemoglobin level, and platelet count)
In the open-label extension phase of ENCORE, 141 of 146 patients (42 patients previously treated with ERT and 99 who continued treatment with Cerdelga) were evaluated for stability, as defined in the initial 12 months of the trial, in clinical parameters (composite of spleen volume, liver volume, hemoglobin level, and platelet count)
![]()
Stability was shown in 120 of 141 (85%) patients at 1 year and 111 of 129 (86%) patients at 2 years of Cerdelga exposure
![]()
91% of patients (42 of 46) treated with Cerdelga for 4 years maintained stability relative to their baseline values for all 4 clinical variables (liver and spleen volumes, hemoglobin level, and platelet count)
![]()
96% of patients (44 of 46) maintained all 4 of these values within prespecified therapeutic goals
Some patients were followed for up to 4 years of Cerdelga treatment. Due to decreasing sample size, results after the 2-year analysis require cautious interpretation.
Cerdelga maintained stability in visceral and hematologic parameters for up to 2 years2,10
LS, least squares.
Exploratory bone data in switch patients8
The ENCORE study was not powered or designed to detect treatment effect of Cerdelga on BMD, and no conclusions may be drawn regarding BMD efficacy, reduction in fracture risk, or other bone pathologies in GD1.
Safety in switch patients
PRIMARY ANALYSIS PERIOD: ADVERSE REACTIONS OCCURRING IN ≥5% OF PATIENTS WITH GD1 SWITCHING FROM ERT TO CERDELGA AND MORE FREQUENTLY THAN IN IMIGLUCERASE2
|
ADVERSE REACTION, n (%) |
Cerdelga (n=106) |
Imiglucerase (n=53) |
| Fatigue | 15 (14) | 1 (2) |
| Headache | 14 (13) | 1 (2) |
| Nausea | 13 (12) | 0 (0) |
| Diarrhea | 13 (12) | 2 (4) |
| Back pain | 13 (12) | 3 (6) |
| Pain in extremity | 12 (11) | 1 (2) |
| Upper abdominal pain | 11 (10) | 0 (0) |
| Dizziness | 8 (9) | 0 (0) |
| Asthenia | 8 (9) | 0 (0) |
| Cough | 7 (7) | 0 (0) |
| Dyspepsia | 7 (7) | 2 (1) |
| Gastroesophageal reflux disease | 7 (7) | 0 (0) |
| Constipation | 5 (5) | 0 (0) |
| Palpitations | 5 (5) | 0 (0) |
| Rash | 5 (5) | 0 (0) |
2% of patients (n=2) given Cerdelga discontinued the study due to adverse events in the primary analysis period9
No new safety signals were observed in the open-label extension period10
Real-world evidence
Results from a retrospective, single-center, observational 20-year study11
In Basiri et al, researchers analyzed 20 years of single-center data on 155 patients with GD1 who were treated with ERT or SRT
This study was observational and not designed to compare the outcomes of treatment or draw conclusions on the effect of one treatment over another
The study found other independent factors for developing osteonecrosis were:
- Patient’s GBA1 genotype
- Residual serum glucosylsphingosine (lyso-GL-1)* level
- History of osteonecrosis prior to initiation of treatment
*Glucosylsphingosine (lyso-GL-1) is the deacylated form of GL-1 and has been used as a highly specific biomarker for Gaucher disease. It has been shown to correlate with clinical disease severity of Gaucher disease.
Dosing and administration2
Cerdelga is available as 84-mg capsules administered once or twice daily. Cerdelga dosing for patients with GD1 is based on their CYP2D6-metabolizer status. Use of Cerdelga may be contraindicated or require dosage adjustment in certain patients based on their CYP2D6-metabolizer status, concomitant use of CYP2D6 or CYP3A inhibitors, and degree of renal or hepatic impairment. Please see full Prescribing Information for additional details.
NDC-58468-0220-1: Carton containing 4 packs of capsules (56 capsules total). Each pack is composed of 1 blister card of 14 capsules and a cardboard wallet.
References: 1. Nalysnyk L, Sugarman R, Cele C, Uyei J, Ward A. Budget impact analysis of eliglustat for the treatment of Gaucher disease type 1 in the United States. J Manag Care Spec Pharm. 2018;24(10):1002-1008. doi:10.18553/jmcp.2018.24.10.1002 2. CERDELGA. Prescribing information. Genzyme Corporation; 2024. 3. Peterschmitt MJ, Cox GF, Ibrahim J, et al. A pooled analysis of adverse events in 393 adults with Gaucher disease type 1 from four clinical trials of oral eliglustat: evaluation of frequency, timing, and duration. Blood Cells Mol Dis. 2018;68:185-191. doi:10.1016/j.bcmd.2017.01.006 4. Hughes DA, Pastores GM. Gaucher disease. In: Adam MP, Feldman J, Mirzaa GM, et al, eds. GeneReviews®. July 27, 2000. Updated December 7, 2023. Accessed March 7, 2024. https://www.ncbi.nlm.nih.gov/books/NBK1269/ 5. Mistry PK, Lukina E, Turkia HB, et al. Effect of oral eliglustat on splenomegaly in patients with Gaucher disease type 1: the ENGAGE randomized clinical trial. JAMA. 2015;313(7):695-706. doi:10.1001/jama.2015.459 6. Mistry PK, Lukina E, Turkia HB, et al. Outcomes after 18 months of eliglustat therapy in treatment-naïve adults with Gaucher disease type 1: the phase 3 ENGAGE trial. Am J Hematol. 2017;92(11):1170-1176. doi:10.1002/ajh.24877 7. Mistry PK, Lukina E, Turkia HB, et al. Clinical outcomes after 4.5 years of eliglustat therapy for Gaucher disease type 1: phase 3 ENGAGE trial final results. Am J Hematol. 2021;96(9):1156-1165. doi:10.1002/ajh.26276 8. Cox TM, Charrow J, Lukina E, Mistry PK, Foster MC, Peterschmitt MJ. Long-term effects of eliglustat on skeletal manifestations in clinical trials of patients with Gaucher disease type 1. Genet Med. 2023;25(2):100329. doi:10.1016/j.gim.2022.10.011 9. Cox TM, Drelichman G, Cravo R, et al. Eliglustat compared with imiglucerase in patients with Gaucher’s disease type 1 stabilised on enzyme replacement therapy: a phase 3, randomised, open-label, non-inferiority trial. Lancet. 2015;385(9985):2355-2362. doi:10.1016/S0140-6736(14)61841-9 10. Cox TM, Drelichman G, Cravo R, et al. Eliglustat maintains long-term clinical stability in patients with Gaucher disease type 1 stabilized on enzyme therapy. Blood. 2017;129(17):2375-2383. doi:10.1182/blood-2016-12-758409 11. Basiri M, Ghaffari ME, Ruan J, et al. Osteonecrosis in Gaucher disease in the era of multiple therapies: biomarker set for risk stratification from a tertiary referral center. Elife. 2023;12:e87537. doi:10.7554/eLife.87537 12. Vpriv. Prescribing information. Takeda Pharmaceuticals U.S.A., Inc.; 2021. 13. Avascular necrosis (osteonecrosis). Cleveland Clinic. Reviewed September 13, 2021. Accessed March 7, 2024. https://my.clevelandclinic.org/health/diseases/14205-avascular-necrosis-osteonecrosis
INDICATION FOR CERDELGA
CERDELGA is indicated for the long-term treatment of adult patients with Gaucher disease type 1 (GD1) who are CYP2D6 extensive metabolizers (EMs), intermediate metabolizers (IMs), or poor metabolizers (PMs) as detected by an FDA-cleared test.
Limitations of Use:
- Patients who are CYP2D6 ultra-rapid metabolizers (URMs) may not achieve adequate concentrations of CERDELGA to achieve a therapeutic effect.
- A specific dosage cannot be recommended for those patients whose CYP2D6 genotype cannot be determined (indeterminate metabolizers).
INDICATION FOR CEREZYME
Cerezyme® (imiglucerase) for injection is indicated for treatment of adults and pediatric patients 2 years of age and older with Type 1 Gaucher disease that results in one or more of the following conditions:
- anemia
- thrombocytopenia
- bone disease
- hepatomegaly or splenomegaly
CONTRAINDICATIONS
CERDELGA is contraindicated in the following patients based on CYP2D6 metabolizer status due to the risk of cardiac arrhythmias from prolongation of the PR, QTc, and/or QRS cardiac intervals:
- Extensive Metabolizers (EMs) taking a strong or moderate CYP2D6 inhibitor concomitantly with a strong or moderate CYP3A inhibitor, EMs with moderate or severe hepatic impairment, or EMs with mild hepatic impairment and taking a strong or moderate CYP2D6 inhibitor.
- Intermediate Metabolizers (IMs) taking a strong or moderate CYP2D6 inhibitor concomitantly with a strong or moderate CYP3A inhibitor, IMs taking a strong CYP3A inhibitor, or IMs with any degree of hepatic impairment.
- Poor Metabolizers (PMs) taking a strong CYP3A inhibitor, or PMs with any degree of hepatic impairment.
WARNINGS AND PRECAUTIONS
CERDELGA is predicted to cause increases in ECG intervals (PR, QTc, and QRS) at substantially elevated plasma concentrations and may increase risk of cardiac arrhythmias. Use of CERDELGA is contraindicated, to be avoided, or requires dosage adjustment in patients taking CYP2D6 or CYP3A inhibitors, depending on CYP2D6 metabolizer status, type of inhibitor, or degree of hepatic impairment. Avoid use of CERDELGA in patients with pre-existing cardiac disease, long QT syndrome, or in combination with Class IA or Class III antiarrhythmic medications.
ADVERSE REACTIONS
The most common adverse reactions (≥10%) to CERDELGA include: fatigue, headache, nausea, diarrhea, back pain, pain in extremities, and upper abdominal pain.
DRUG INTERACTIONS
Coadministration of CERDELGA with CYP2D6 or CYP3A inhibitors may increase eliglustat concentrations, which may increase the risk of cardiac arrhythmias from prolongations of the PR, QTc, and/or QRS cardiac interval. Use of CERDELGA is contraindicated, to be avoided, or may require dosage adjustment depending on the concomitant drug and CYP2D6 metabolizer status. See section 7 of the full Prescribing Information for more details and other potentially significant drug interactions.
USE IN SPECIFIC POPULATIONS
Available data on the use of CERDELGA in pregnant women is not sufficient to assess drug-associated risks of major birth defects, miscarriage, or adverse maternal or fetal outcomes. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for CERDELGA and any potential adverse effects on the breastfed child from CERDELGA or from the underlying maternal condition.
Use of CERDELGA in patients with renal impairment is based on the patient’s CYP2D6 metabolizer status. Avoid use of CERDELGA in EMs with end-stage renal disease (ESRD), and IMs and PMs with any degree of renal impairment.
Use of CERDELGA is contraindicated or may require dosage adjustment in patients with hepatic impairment based on CYP2D6 metabolizer status, concomitant use of CYP2D6 or CYP3A inhibitors, and degree of hepatic impairment.
Please see accompanying full Prescribing Information.
IMPORTANT SAFETY INFORMATION FOR CEREZYME
Patients treated with enzyme replacement therapies have experienced life-threatening hypersensitivity reactions, including anaphylaxis. Anaphylaxis has occurred during the early course of enzyme replacement therapy and after extended duration of therapy.
Initiate CEREZYME in a healthcare setting with appropriate medical monitoring and support measures, including access to cardiopulmonary resuscitation equipment. If a severe hypersensitivity reaction (e.g., anaphylaxis) occurs, discontinue CEREZYME and immediately initiate appropriate medical treatment, including use of epinephrine. Inform patients of the symptoms of life-threatening hypersensitivity reactions, including anaphylaxis, and to seek immediate medical care should symptoms occur.
Warnings and Precautions:
Hypersensitivity Reactions Including Anaphylaxis: See Boxed WARNING.
Patients with antibody to imiglucerase have a higher risk of hypersensitivity reactions. Consider periodic monitoring during the first year of treatment for IgG antibody formation.
Consider risks and benefits of readministering Cerezyme to individual patients following a severe reaction. Consider reducing the rate of infusion, pretreat with antihistamines and/or corticosteroids, and monitor patients for new signs and symptoms of a severe hypersensitivity reaction.
Infusion-Associated Reactions:
Infusion associated reactions (IARs) have been observed in patients treated with Cerezyme. If an IAR occurs, decreasing the infusion rate, temporarily stopping the infusion and/or administering antihistamines and/or antipyretics may ameliorate the symptoms. Closely monitor patients who have experienced IARs when re-administering Cerezyme.
Adverse Reactions:
- Adverse reactions reported in adults include back pain, chills, dizziness, fatigue, headache, hypersensitivity reactions, nausea, pyrexia, and vomiting.
- Adverse reactions reported in pediatric patients 2 years of age and older are similar to adults.
Please see Full Prescribing Information, including Boxed WARNING.
INDICATION FOR CERDELGA
CERDELGA is indicated for the long-term treatment of adult patients with Gaucher disease type 1 (GD1) who are CYP2D6 extensive metabolizers (EMs), intermediate metabolizers (IMs), or poor metabolizers (PMs) as detected by an FDA-cleared test.
Limitations of Use:
- Patients who are CYP2D6 ultra-rapid metabolizers (URMs) may not achieve adequate concentrations of CERDELGA to achieve a therapeutic effect.
- A specific dosage cannot be recommended for those patients whose CYP2D6 genotype cannot be determined (indeterminate metabolizers).
INDICATION FOR CEREZYME
Cerezyme® (imiglucerase) for injection is indicated for treatment of adults and pediatric patients 2 years of age and older with Type 1 Gaucher disease that results in one or more of the following conditions:
- anemia
- thrombocytopenia
- bone disease
- hepatomegaly or splenomegaly
CONTRAINDICATIONS
CERDELGA is contraindicated in the following patients based on CYP2D6 metabolizer status due to the risk of cardiac arrhythmias from prolongation of the PR, QTc, and/or QRS cardiac intervals:
- Extensive Metabolizers (EMs) taking a strong or moderate CYP2D6 inhibitor concomitantly with a strong or moderate CYP3A inhibitor, EMs with moderate or severe hepatic impairment, or EMs with mild hepatic impairment and taking a strong or moderate CYP2D6 inhibitor.
- Intermediate Metabolizers (IMs) taking a strong or moderate CYP2D6 inhibitor concomitantly with a strong or moderate CYP3A inhibitor, IMs taking a strong CYP3A inhibitor, or IMs with any degree of hepatic impairment.
- Poor Metabolizers (PMs) taking a strong CYP3A inhibitor, or PMs with any degree of hepatic impairment.
WARNINGS AND PRECAUTIONS
CERDELGA is predicted to cause increases in ECG intervals (PR, QTc, and QRS) at substantially elevated plasma concentrations and may increase risk of cardiac arrhythmias. Use of CERDELGA is contraindicated, to be avoided, or requires dosage adjustment in patients taking CYP2D6 or CYP3A inhibitors, depending on CYP2D6 metabolizer status, type of inhibitor, or degree of hepatic impairment. Avoid use of CERDELGA in patients with pre-existing cardiac disease, long QT syndrome, or in combination with Class IA or Class III antiarrhythmic medications.
ADVERSE REACTIONS
The most common adverse reactions (≥10%) to CERDELGA include: fatigue, headache, nausea, diarrhea, back pain, pain in extremities, and upper abdominal pain.
DRUG INTERACTIONS
Coadministration of CERDELGA with CYP2D6 or CYP3A inhibitors may increase eliglustat concentrations, which may increase the risk of cardiac arrhythmias from prolongations of the PR, QTc, and/or QRS cardiac interval. Use of CERDELGA is contraindicated, to be avoided, or may require dosage adjustment depending on the concomitant drug and CYP2D6 metabolizer status. See section 7 of the full Prescribing Information for more details and other potentially significant drug interactions.
USE IN SPECIFIC POPULATIONS
Available data on the use of CERDELGA in pregnant women is not sufficient to assess drug-associated risks of major birth defects, miscarriage, or adverse maternal or fetal outcomes. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for CERDELGA and any potential adverse effects on the breastfed child from CERDELGA or from the underlying maternal condition.
Use of CERDELGA in patients with renal impairment is based on the patient’s CYP2D6 metabolizer status. Avoid use of CERDELGA in EMs with end-stage renal disease (ESRD), and IMs and PMs with any degree of renal impairment.
Use of CERDELGA is contraindicated or may require dosage adjustment in patients with hepatic impairment based on CYP2D6 metabolizer status, concomitant use of CYP2D6 or CYP3A inhibitors, and degree of hepatic impairment.
Please see accompanying full Prescribing Information.
IMPORTANT SAFETY INFORMATION FOR CEREZYME
Patients treated with enzyme replacement therapies have experienced life-threatening hypersensitivity reactions, including anaphylaxis. Anaphylaxis has occurred during the early course of enzyme replacement therapy and after extended duration of therapy.
Initiate CEREZYME in a healthcare setting with appropriate medical monitoring and support measures, including access to cardiopulmonary resuscitation equipment. If a severe hypersensitivity reaction (e.g., anaphylaxis) occurs, discontinue CEREZYME and immediately initiate appropriate medical treatment, including use of epinephrine. Inform patients of the symptoms of life-threatening hypersensitivity reactions, including anaphylaxis, and to seek immediate medical care should symptoms occur.
Warnings and Precautions:
Hypersensitivity Reactions Including Anaphylaxis: See Boxed WARNING.
Patients with antibody to imiglucerase have a higher risk of hypersensitivity reactions. Consider periodic monitoring during the first year of treatment for IgG antibody formation.
Consider risks and benefits of readministering Cerezyme to individual patients following a severe reaction. Consider reducing the rate of infusion, pretreat with antihistamines and/or corticosteroids, and monitor patients for new signs and symptoms of a severe hypersensitivity reaction.
Infusion-Associated Reactions:
Infusion associated reactions (IARs) have been observed in patients treated with Cerezyme. If an IAR occurs, decreasing the infusion rate, temporarily stopping the infusion and/or administering antihistamines and/or antipyretics may ameliorate the symptoms. Closely monitor patients who have experienced IARs when re-administering Cerezyme.
Adverse Reactions:
- Adverse reactions reported in adults include back pain, chills, dizziness, fatigue, headache, hypersensitivity reactions, nausea, pyrexia, and vomiting.
- Adverse reactions reported in pediatric patients 2 years of age and older are similar to adults.
Please see Full Prescribing Information, including Boxed WARNING.
INDICATION FOR CERDELGA
CERDELGA is indicated for the long-term treatment of adult patients with Gaucher disease type 1 (GD1) who are CYP2D6 extensive metabolizers (EMs), intermediate metabolizers (IMs), or poor metabolizers (PMs) as detected by an FDA-cleared test.
Limitations of Use:
- Patients who are CYP2D6 ultra-rapid metabolizers (URMs) may not achieve adequate concentrations of CERDELGA to achieve a therapeutic effect.
- A specific dosage cannot be recommended for those patients whose CYP2D6 genotype cannot be determined (indeterminate metabolizers).
INDICATION FOR CEREZYME
Cerezyme® (imiglucerase) for injection is indicated for treatment of adults and pediatric patients 2 years of age and older with Type 1 Gaucher disease that results in one or more of the following conditions:
- anemia
- thrombocytopenia
- bone disease
- hepatomegaly or splenomegaly
CONTRAINDICATIONS
CERDELGA is contraindicated in the following patients based on CYP2D6 metabolizer status due to the risk of cardiac arrhythmias from prolongation of the PR, QTc, and/or QRS cardiac intervals:
- Extensive Metabolizers (EMs) taking a strong or moderate CYP2D6 inhibitor concomitantly with a strong or moderate CYP3A inhibitor, EMs with moderate or severe hepatic impairment, or EMs with mild hepatic impairment and taking a strong or moderate CYP2D6 inhibitor.
- Intermediate Metabolizers (IMs) taking a strong or moderate CYP2D6 inhibitor concomitantly with a strong or moderate CYP3A inhibitor, IMs taking a strong CYP3A inhibitor, or IMs with any degree of hepatic impairment.
- Poor Metabolizers (PMs) taking a strong CYP3A inhibitor, or PMs with any degree of hepatic impairment.
WARNINGS AND PRECAUTIONS
CERDELGA is predicted to cause increases in ECG intervals (PR, QTc, and QRS) at substantially elevated plasma concentrations and may increase risk of cardiac arrhythmias. Use of CERDELGA is contraindicated, to be avoided, or requires dosage adjustment in patients taking CYP2D6 or CYP3A inhibitors, depending on CYP2D6 metabolizer status, type of inhibitor, or degree of hepatic impairment. Avoid use of CERDELGA in patients with pre-existing cardiac disease, long QT syndrome, or in combination with Class IA or Class III antiarrhythmic medications.
ADVERSE REACTIONS
The most common adverse reactions (≥10%) to CERDELGA include: fatigue, headache, nausea, diarrhea, back pain, pain in extremities, and upper abdominal pain.
DRUG INTERACTIONS
Coadministration of CERDELGA with CYP2D6 or CYP3A inhibitors may increase eliglustat concentrations, which may increase the risk of cardiac arrhythmias from prolongations of the PR, QTc, and/or QRS cardiac interval. Use of CERDELGA is contraindicated, to be avoided, or may require dosage adjustment depending on the concomitant drug and CYP2D6 metabolizer status. See section 7 of the full Prescribing Information for more details and other potentially significant drug interactions.
USE IN SPECIFIC POPULATIONS
Available data on the use of CERDELGA in pregnant women is not sufficient to assess drug-associated risks of major birth defects, miscarriage, or adverse maternal or fetal outcomes. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for CERDELGA and any potential adverse effects on the breastfed child from CERDELGA or from the underlying maternal condition.
Use of CERDELGA in patients with renal impairment is based on the patient’s CYP2D6 metabolizer status. Avoid use of CERDELGA in EMs with end-stage renal disease (ESRD), and IMs and PMs with any degree of renal impairment.
Use of CERDELGA is contraindicated or may require dosage adjustment in patients with hepatic impairment based on CYP2D6 metabolizer status, concomitant use of CYP2D6 or CYP3A inhibitors, and degree of hepatic impairment.
Please see accompanying full Prescribing Information.
IMPORTANT SAFETY INFORMATION FOR CEREZYME
Patients treated with enzyme replacement therapies have experienced life-threatening hypersensitivity reactions, including anaphylaxis. Anaphylaxis has occurred during the early course of enzyme replacement therapy and after extended duration of therapy.
Initiate CEREZYME in a healthcare setting with appropriate medical monitoring and support measures, including access to cardiopulmonary resuscitation equipment. If a severe hypersensitivity reaction (e.g., anaphylaxis) occurs, discontinue CEREZYME and immediately initiate appropriate medical treatment, including use of epinephrine. Inform patients of the symptoms of life-threatening hypersensitivity reactions, including anaphylaxis, and to seek immediate medical care should symptoms occur.
Warnings and Precautions:
Hypersensitivity Reactions Including Anaphylaxis: See Boxed WARNING.
Patients with antibody to imiglucerase have a higher risk of hypersensitivity reactions. Consider periodic monitoring during the first year of treatment for IgG antibody formation.
Consider risks and benefits of readministering Cerezyme to individual patients following a severe reaction. Consider reducing the rate of infusion, pretreat with antihistamines and/or corticosteroids, and monitor patients for new signs and symptoms of a severe hypersensitivity reaction.
Infusion-Associated Reactions:
Infusion associated reactions (IARs) have been observed in patients treated with Cerezyme. If an IAR occurs, decreasing the infusion rate, temporarily stopping the infusion and/or administering antihistamines and/or antipyretics may ameliorate the symptoms. Closely monitor patients who have experienced IARs when re-administering Cerezyme.
Adverse Reactions:
- Adverse reactions reported in adults include back pain, chills, dizziness, fatigue, headache, hypersensitivity reactions, nausea, pyrexia, and vomiting.
- Adverse reactions reported in pediatric patients 2 years of age and older are similar to adults.
Please see Full Prescribing Information, including Boxed WARNING.
This site is intended for US payers only.
© Sanofi. All rights reserved.
Cerdelga and Sanofi are registered trademarks of Sanofi or an affiliate.
CareConnect Personalized Support Services is a trademark of Sanofi or an affiliate.