Rare blood disorders
FDA-approved products in rare blood disorders
Protection your way: Sanofi’s commitment to personalized care for hemophilia A and B
A tailored therapeutic approach for hemophilia A and B, aimed at minimizing treatment burden and enhancing disease management
In the United States, approximately 30,000 to 33,000 males have hemophilia A or B, with over 40% having a severe form of the condition.1*
Patients with hemophilia have insufficient clotting factor proteins, resulting in inadequate thrombin generation, leading to excessive and longer-than-usual bleeding after physical injury. Individuals with hemophilia may also experience spontaneous bleeding into their muscles, organs, and joints.2-4
Explore Sanofi’s comprehensive portfolio designed to support individualized treatment goals for patients with hemophilia A and B—including those with inhibitors
![]()
Learn about our FDA-approved products for hemophilia A, designed to deliver effective protection.
![]()
Learn about our FDA-approved product for hemophilia B, featuring an extended half-life.

Learn about our FDA-approved product for hemophilia A and B, subcutaneous therapy for patients with or without inhibitors.
Acquired thrombotic thrombocytopenic purpura (aTTP)
aTTP is a rare, life-threatening blood disorder characterized by the formation of small blood clots (microthrombi) in the body’s smallest blood vessels, leading to low platelet count (thrombocytopenia), destruction of red blood cells (hemolytic anemia), and organ damage.5-7
*Although the bleeding disorder primarily affects males, females can also have hemophilia.2
†Approved in 2023.
‡Approved in 2014.
Exclusive near-term research in immune thrombocytopenia (ITP)
ITP is a heterogenous autoimmune disease characterized by isolated thrombocytopenia8

There are 3 distinct disease phases: newly diagnosed (within 3 months of diagnosis), persistent (between 3 and 12 months after diagnosis), and chronic (lasting 12 months or more after diagnosis).9
Bleeding occurs in ~60% of patients and significantly impacts quality of life.10,11
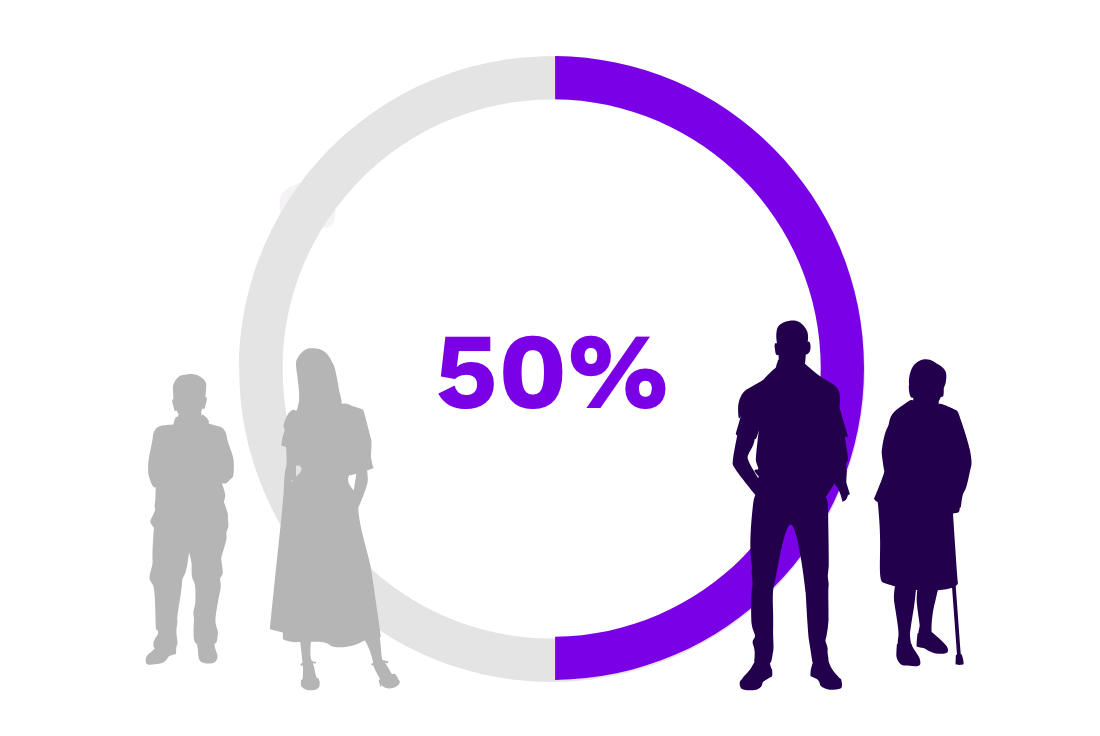
Half of patients with persistent or chronic ITP require treatment.12
The heterogenous nature of ITP creates the need for multiple treatment options
There is an unmet need for an effective treatment option that has a positive impact on patient quality of life and a favorable safety profile.
Explore our other areas of focus
Immunology and
inflammation
Neurology

Oncology
Rare diseases

Search for other FDA-approved products
FDA, US Food and Drug Administration.
References: 1. Soucie JM, Miller CH, Dupervil B, Le B, Buckner TW. Occurrence rates of haemophilia among males in the United States based on surveillance conducted in specialized haemophilia treatment centres. Haemophilia. 2020;26(3):487-493. doi:10.1111/hae.13998 2. Women can have hemophilia, too. Centers for Disease Control and Prevention. Accessed June 10, 2025. https://archive.cdc.gov/#/details?url=https://www.cdc.gov/ncbddd/hemophilia/features/women-and-hemophilia.html 3. Sidonio RF Jr, Homan M, Kenet G, Dargaud Y. Thrombin generation and implications for hemophilia therapies: a narrative review. Res Pract Thromb Haemost. 2023;7(1):e100018. doi:10.1016/j.rpth.2022.100018 4. A new study of hemophilia occurrence finds many more cases in the United States. Centers for Disease Control and Prevention. Accessed June 10, 2025. https://archive.cdc.gov/#/details?url=https:// www.cdc.gov/ncbddd/hemophilia/features/keyfinding-hemophilia-occurrence-US.html 5. Goel R, King KE, Takemoto CM, Ness PM, Tobian AAR. Prognostic risk-stratified score for predicting mortality in hospitalized patients with thrombotic thrombocytopenic purpura: nationally representative data from 2007 to 2012. Transfusion. 2016;56(6):1451-1458. doi:10.1111/trf.13586 6. Kremer Hovinga JA, Vesely SK, Terrell DR, Lämmle B, George JN. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2010;115(8):1500-1511. doi:10.1182/blood-2009-09-243790 7. Peyvandi F, Scully M, Kremer Hovinga JA, et al. Caplacizumab reduces the frequency of major thromboembolic events, exacerbations and death in patients with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2017;15(7):1448-1452. doi:10.1111/jth.13716 8. Cooper N, Ghanima W. Immune thrombocytopenia. N Engl J Med. 2019;381(10):945-955. doi:10.1056/NEJMcp1810479 9. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393. doi:10.1182/blood-2008-07-162503 10. Audia S, Bonnotte B. Emerging therapies in immune thrombocytopenia. J Clin Med. 2021;10(5):1004. doi:10.3390/jcm10051004 11. Piel-Julian ML, Mahévas M, Germain J, et al. Risk factors for bleeding, including platelet count threshold, in newly diagnosed immune thrombocytopenia adults. J Thromb Haemost. 2018;16(9):1830-1842. doi:10.1111/jth.14227 12. Data on file. Sanofi. 2025.
This site is intended for US payers only.
© 2025 Sanofi. All rights reserved.