DUPIXENT® in uncontrolled moderate-to-severe atopic dermatitis (AD)
Patients aged 6+ months
THE FIRST AND ONLY BIOLOGIC APPROVED FOR UNCONTROLLED MODERATE-TO-SEVERE AD IN PATIENTS AS YOUNG AS 6 MONTHS1
DUPIXENT WAS STUDIED ACROSS A WIDE RANGE OF AGE GROUPS1-4
Adults aged
18 years and older
Adolescents aged
12 to 17 years
Children aged
6 to 11 years
Infants to
preschoolers aged
6 months to 5 years
Efficacy in AD: Adult patients
DUPIXENT provides rapid itch relief and sustained skin clearance for patients aged ≥18 years with uncontrolled moderate-to-severe AD.
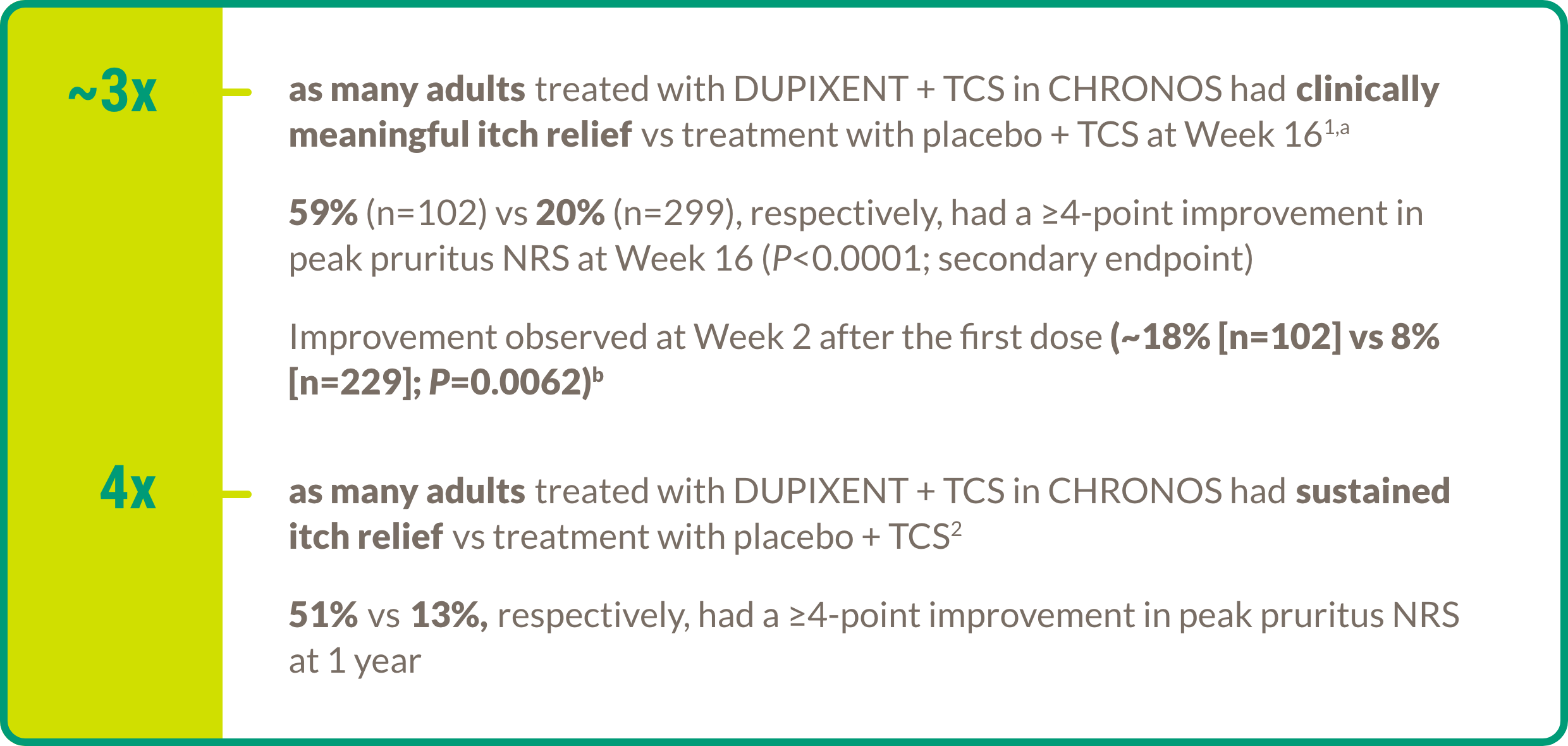
Itch relief
aAnalysis for this endpoint was performed for patients with a baseline peak pruritus NRS score ≥4.1
bData from the dupilumab 300 mg Q2W + TCS group (n=106).
Skin clearance
aDefinitive conclusions cannot be made at Week 2, as this was a post-hoc analysis in which data were not multiplicity controlled and P value was nominal.1
EASI, Eczema Area and Severity Index; IGA, Investigator’s Global Assessment Scale; NRS, numerical rating scale; Q2W, once every 2 weeks; TCS, topical corticosteroid.
Efficacy in AD: Adolescent and pediatric patients1
DUPIXENT is the first and only biologic approved to treat uncontrolled moderate-to-severe AD from infancy to adulthood (aged 6 months and older).1
Itch relief at Week 16
aDefined according to the peak pruritus NRS score (≥4-point improvement).2
bDefined according to the worst scratch/itch NRS score (≥4-point improvement).2
Skin clearance at Week 16
aDefined as an IGA score of 0 or 1.2
![]()
DUPIXENT was studied in over 3000 (800 pediatric) moderate-to-severe AD patients across 7 pivotal trials that included monotherapy and concomitant TCS1
![]()
In AD, clinically meaningful improvement was noted at Week 16 in monotherapy and concomitant TCS trials in adults and pediatric patients (aged ≥6 months)1
Q2W, once every 2 weeks; Q4W, once every 4 weeks.
Safety profile in AD: Adult patients
DUPIXENT has a demonstrated safety profile in adults with uncontrolled moderate-to-severe AD.1
In adults (aged ≥18 years), the safety profile of DUPIXENT + TCS through Week 52 was generally consistent with that observed at Week 16
ADVERSE REACTIONS OCCURRING IN >1% OF ADULT PATIENTS THROUGH WEEK 16
| DUPIXENT MONOTHERAPYa | DUPIXENT + TCSb | |||
| ADVERSE REACTION | DUPIXENTc N=529 n (%) |
Placebo N=517 n (%) |
DUPIXENT + TCSc N=110 n (%) |
Placebo + TCS N=315 n (%) |
| Injection site reaction | 51 (10) | 28 (5) | 11 (10) | 18 (6) |
| Conjunctivitisd | 51 (10) | 12 (2) | 10 (9) | 15 (5) |
| Blepharitis | 2 (<1) | 1 (<1) | 5 (5) | 2 (1) |
| Oral herpes | 20 (4) | 8 (2) | 3 (3) | 5 (2) |
| Keratitise | 1 (<1) | 0 | 4 (4) | 0 |
| Eye pruritus | 3 (1) | 1 (<1) | 2 (2) | 2 (1) |
| Other HSV infectionf | 10 (2) | 6 (1) | 1 (1) | 1 (<1) |
| Dry eye | 1 (<1) | 0 | 2 (2) | 1 (<1) |
Treatment-emergent eosinophilia (≥5000 cells/μL) was reported in <3% of DUPIXENT-treated subjects and <0.5% of placebo-treated subjects (SOLO 1, SOLO 2, and AD-1021; DRI12544, QUEST, and VOYAGE; SINUS-24 and SINUS-52; PRIME and PRIME-2) and 8% of DUPIXENT-treated subjects and 0% of placebo-treated subjects (AD-1539).g
aPooled analysis of SOLO 1, SOLO 2, and AD-1021 (Phase 2 dose-ranging study).1
bAnalysis of CHRONOS in which patients were on background TCS therapy.1
cDUPIXENT 600 mg at Week 0, followed by 300 mg every 2 weeks.1
dConjunctivitis cluster includes conjunctivitis, allergic conjunctivitis, bacterial conjunctivitis, viral conjunctivitis, giant papillary conjunctivitis, eye irritation, and eye inflammation.1
eKeratitis cluster includes keratitis, ulcerative keratitis, allergic keratitis, atopic keratoconjunctivitis, and ophthalmic herpes simplex.
fOther herpes simplex infection cluster includes herpes simplex, genital herpes, herpes simplex otitis externa, and herpes virus infection but excludes eczema herpeticum.1
gDRI12544, QUEST, and VOYAGE are part of the asthma clinical trial program; SINUS-24 and SINUS-52 are part of the CRSwNP clinical trial program; PRIME and PRIME-2 are part of the PN clinical trial program.1
CRSwNP, chronic rhinosinusitis with nasal polyposis; HSV, herpes simplex virus; PN, prurigo nodularis.
Long-term safety profile in adults6
The long-term safety profile observed in the AD-1225 open-label extension (OLE) study through 260 weeks was generally consistent with the safety profile of DUPIXENT observed in controlled studies.
Safety profile in AD: Adolescent and pediatric patients1
The safety profile in pediatric patients through Week 16 in pivotal trials was similar to that in adults with AD.
Treatment-emergent eosinophilia (>5000 cells/microliter) was reported in 8% of DUPIXENT-treated subjects and 0% of placebo-treated subjects (AD-1539, age 6 months to 5 years).
In an OLE study (AD-1434), the long-term safety profile of DUPIXENT ± TCS in pediatric patients observed through Week 52 was consistent with that observed in adults, with hand-foot-and-mouth disease and skin papilloma (incidence >2%) reported in patients aged 6 months to 5 years. These cases did not lead to study drug discontinuation.
Real-world experience in AD
Patient persistence7
In adult patients aged 18 years and older with moderate-to-severe AD treated with DUPIXENT (n=1963):
![]()
92% remained on therapy at 6 months and 77% remained on therapy at 1 year
![]()
79% of patients who discontinued DUPIXENT re-initiated within 4 months
Study design: From IBM MarketScan Commercial and Medicare supplemental databases: 1963 adults with ≥1 DUPIXENT prescription(s) were identified between March 28, 2017, and March 31, 2018, with continuous enrollment during the baseline period. Patients were followed from their first prescription until July 31, 2018, or disenrollment. Kaplan-Meier curves were used to estimate persistence at 6 and 12 months using a 30-day grace period and assuming a 14-day injection frequency.2
Limitations of analysis: Limitations of this study may be the potential misclassification of persistence due to assumptions regarding the injection frequency and grace period. The study includes early adopters of DUPIXENT; therefore, as utilization in clinical practice increases over time, further analyses are recommended to confirm these findings. Differences exist in patient populations and data collection methods vs randomized controlled trials.2
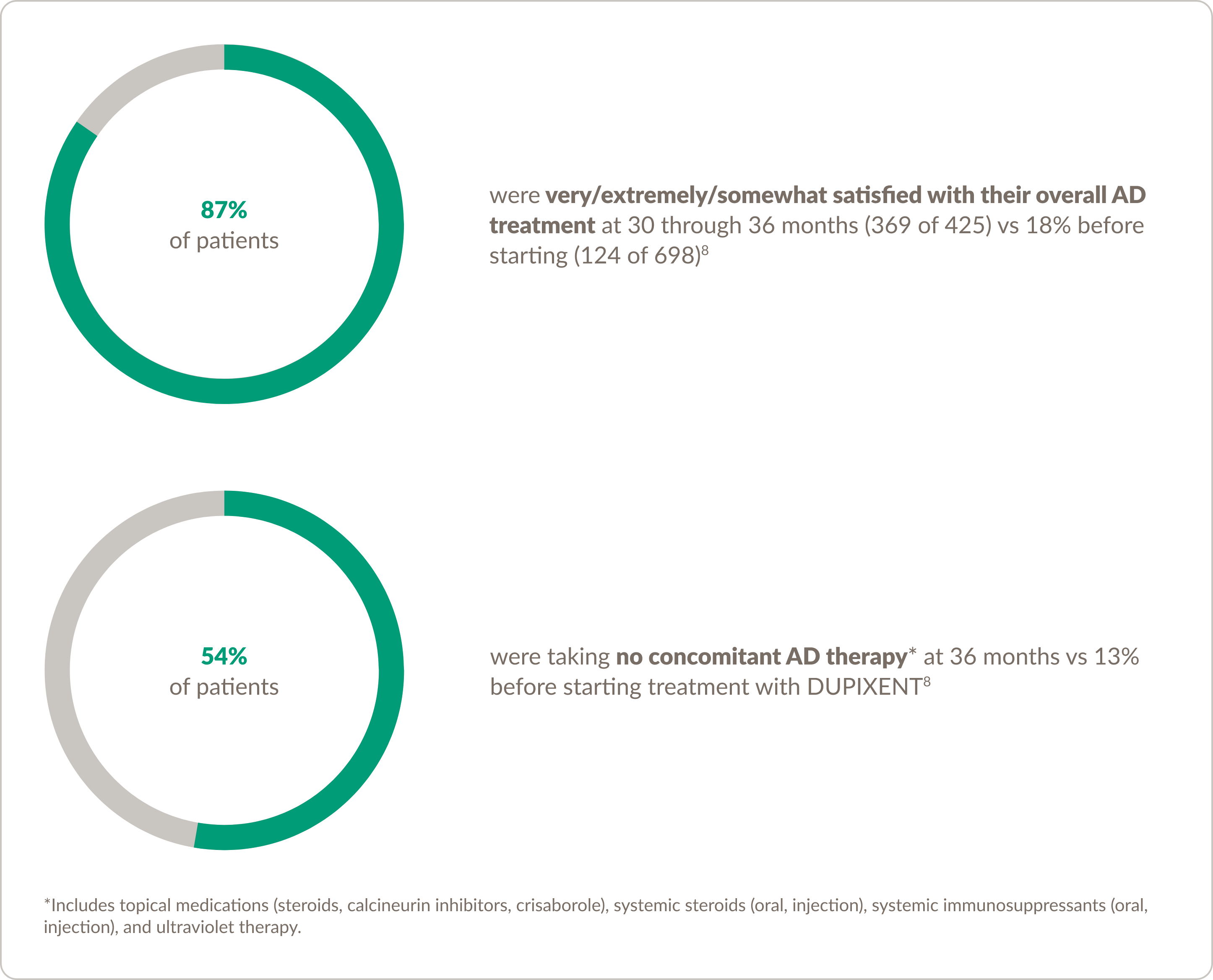
Patient satisfaction and concomitant treatment use
A survey of adult patients treated with DUPIXENT8 found that:
Study design: Based on a prospective, longitudinal patient survey of 699 adult patients who had not been treated with DUPIXENT prior to this study. Surveys were completed at baseline (prior to DUPIXENT treatment) and at Months 1, 2, 3, 6, 9, 12, and 30 to 36 after initiation of DUPIXENT. Data are from an interim analysis of patients who had completed surveys at 1 month (n=632) through 30 to 36 months (n=425). Patient-reported satisfaction with current AD treatment was evaluated using the question “How satisfied are you with your current treatment(s) for AD?”, with responses on a 7-point Likert scale ranging from “extremely satisfied” to “extremely dissatisfied.”2
Limitations of analysis: The survey data were collected from patients who enrolled in the DUPIXENT patient support program, and these patients may have different perceptions of DUPIXENT vs those who did not enroll. Patients who continue to respond to long-term surveys may be self-selecting for favorable effectiveness and safety. There was also potential for recall bias because outcomes were self-reported, and observed data may bias results. Safety and tolerability data were not collected.2
Relieve-AD: Long-term data9
Any prescription topical medications include calcineurin inhibitor creams or ointments (eg, pimecrolimus, tacrolimus), crisaborole ointment, and steroid creams or ointments (eg, hydrocortisone); any systemic steroid use was defined as the use of steroid pills or injections (eg, prednisone, prednisolone, dexamethasone, methylprednisolone); any systemic immunosuppressant use was defined as the use of oral systemic immunosuppressants (excluding steroid pills; eg, mycophenolate mofetil, azathioprine, cyclosporine, tacrolimus) or CellCept injections.
Study design: Based on a prospective, longitudinal patient survey of 699 adult patients
who had not been treated with DUPIXENT prior to this study. Surveys were completed at baseline (prior to DUPIXENT treatment) and at Months 1, 2, 3, 6, 9, 12, and 30 to 36 after initiation of DUPIXENT. Data are from an interim analysis of patients who had completed surveys at 1 month (n=632) through 30 to 36 months (n=425). Patient-reported satisfaction with current AD treatment was evaluated using the question “How satisfied are you with your current treatment(s) for AD?”, with responses on a 7-point Likert scale ranging from “extremely satisfied” to “extremely dissatisfied.”2
Limitations of analysis: The survey data were collected from patients who enrolled in the DUPIXENT patient support program, and these patients may have different perceptions of DUPIXENT vs those who did not enroll. Patients who continue to respond to long-term surveys may be self-selecting for favorable effectiveness and safety. There was also potential for recall bias because outcomes were self-reported, and observed data may bias results. Safety and tolerability data were not collected.2
Explore the other indications of DUPIXENT
Moderate-to-severe eosinophilic or OCS-dependent asthma
(aged 6+ years)
Inadequately controlled chronic rhinosinusitis with nasal polyps (aged 12+ years)
References: 1. DUPIXENT. Prescribing information. Regeneron Pharmaceuticals, Inc.; 2024. 2. Data on file. Sanofi US. 3. Oakley A. EASI score. DermNet. Updated January 2015. Accessed July 24, 2024. https://dermnetnz.org/topics/easi-score 4. Paller AS, Simpson EL, Siegfried EC, et al. Dupilumab in children aged 6 months to younger than 6 years with uncontrolled atopic dermatitis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2022;400(10356):908-919. doi:10.1016/S0140-6736(22)01539-2 5. Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287-2303. doi:10.1016/S0140-6736(17)31191-1 6. Beck LA, Bissonnette R, Deleuran M, et al. Safety of long-term dupilumab treatment in adults with moderate-to-severe atopic dermatitis: results from a 5-year open-label extension trial. Poster presented at: 5th Annual Revolutionizing Atopic Dermatitis (RAD) Conference; April 29-May 1, 2023; Washington DC. 7. Silverberg JI, Guttman-Yassky E, Gadkari A, et al. Real-world persistence with dupilumab among adults with atopic dermatitis. Ann Allergy Asthma Immunol. 2021;126(1):40-45. doi:10.1016/j.anai.2020.07.026 8. Strober B, Chao J, Chuang CC. Early and sustained improvement in atopic dermatitis (AD) disease control and treatment satisfaction with dupilumab in clinical practice: long-term data from the RELIEVE-AD study. Poster presented at: American Academy of Dermatology (AAD) 2022 Annual Meeting; March 25-29, 2022; Boston, MA. 9. Delevry D, Chao J, Chuang CC. Dupilumab decreases concomitant therapy use in adults with atopic dermatitis (AD): long-term data from the RELIEVE-AD study. Poster presented at: American Academy of Dermatology (AAD) 2022 Annual Meeting; March 25–29, 2022; Boston, MA.
INDICATIONS
Atopic Dermatitis: DUPIXENT is indicated for the treatment of adult and pediatric patients aged 6 months and older with moderate-to-severe atopic dermatitis (AD) whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. DUPIXENT can be used with or without topical corticosteroids.
Asthma: DUPIXENT is indicated as an add-on maintenance treatment of adult and pediatric patients aged 6 years and older with moderate-to-severe asthma characterized by an eosinophilic phenotype or with oral corticosteroid dependent asthma. Limitations of Use: DUPIXENT is not indicated for the relief of acute bronchospasm or status asthmaticus.
Chronic Rhinosinusitis with Nasal Polyps: DUPIXENT is indicated as an add-on maintenance treatment in adult and pediatric patients aged 12 years and older with inadequately controlled chronic rhinosinusitis with nasal polyps (CRSwNP).
Eosinophilic Esophagitis: DUPIXENT is indicated for the treatment of adult and pediatric patients aged 1 year and older, weighing at least 15 kg, with eosinophilic esophagitis (EoE).
Prurigo Nodularis: DUPIXENT is indicated for the treatment of adult patients with prurigo nodularis (PN).
Chronic Obstructive Pulmonary Disease: DUPIXENT is indicated as an add-on maintenance treatment of adult patients with inadequately controlled chronic obstructive pulmonary disease (COPD) and an eosinophilic phenotype. Limitations of Use: DUPIXENT is not indicated for the relief of acute bronchospasm.
Chronic Spontaneous Urticaria: DUPIXENT is indicated for the treatment of adult and pediatric patients aged 12 years and older with chronic spontaneous urticaria (CSU) who remain symptomatic despite H1 antihistamine treatment. Limitations of Use: DUPIXENT is not indicated for treatment of other forms of urticaria.
CONTRAINDICATION: DUPIXENT is contraindicated in patients with known hypersensitivity to dupilumab or any of its excipients.
WARNINGS AND PRECAUTIONS
Hypersensitivity: Hypersensitivity reactions, including anaphylaxis, serum sickness or serum sickness-like reactions, angioedema, generalized urticaria, rash, erythema nodosum, and erythema multiforme have been reported. If a clinically significant hypersensitivity reaction occurs, institute appropriate therapy and discontinue DUPIXENT.
Conjunctivitis and Keratitis: Conjunctivitis and keratitis occurred more frequently in AD subjects who received DUPIXENT versus placebo, with conjunctivitis being the most frequently reported eye disorder. Conjunctivitis also occurred more frequently in adult CRSwNP subjects, PN subjects, and COPD subjects who received DUPIXENT compared to those who received placebo. Conjunctivitis and keratitis have been reported with DUPIXENT in postmarketing settings, predominantly in AD patients. Some patients reported visual disturbances (e.g., blurred vision) associated with conjunctivitis or keratitis. Advise patients or their caregivers to report new-onset or worsening eye symptoms. Consider ophthalmological examination for patients who develop conjunctivitis that does not resolve following standard treatment or signs and symptoms suggestive of keratitis, as appropriate.
Eosinophilic Conditions: Patients being treated for asthma may present with clinical features of eosinophilic pneumonia or eosinophilic granulomatosis with polyangiitis (EGPA). These events may be associated with the reduction of oral corticosteroid therapy. Healthcare providers should be alert to vasculitic rash, worsening pulmonary symptoms, cardiac complications, kidney injury, and/or neuropathy presenting in their patients with eosinophilia. Cases of eosinophilic pneumonia were reported in adults who participated in the asthma development program and cases of EGPA have been reported with DUPIXENT in adults who participated in the asthma development program as well as in adults with co-morbid asthma in the CRSwNP development program. Advise patients to report signs of eosinophilic pneumonia and EGPA. Consider withholding DUPIXENT if eosinophilic pneumonia or EGPA are suspected.
Acute Symptoms of Asthma or Chronic Obstructive Pulmonary Disease or Acute Deteriorating Disease: Do not use DUPIXENT to treat acute symptoms or acute exacerbations of asthma or COPD, acute bronchospasm, or status asthmaticus. Patients should seek medical advice if their asthma or COPD remains uncontrolled or worsens after initiation of DUPIXENT.
Risk Associated with Abrupt Reduction of Corticosteroid Dosage: Do not discontinue systemic, topical, or inhaled corticosteroids abruptly upon initiation of DUPIXENT. Reductions in corticosteroid dose, if appropriate, should be gradual and performed under the direct supervision of a healthcare provider. Reduction in corticosteroid dose may be associated with systemic withdrawal symptoms and/or unmask conditions previously suppressed by systemic corticosteroid therapy.
Patients with Co-morbid Asthma: Advise patients with co-morbid asthma not to adjust or stop their asthma treatments without consultation with their physicians.
Psoriasis: Cases of new-onset psoriasis have been reported with the use of DUPIXENT for the treatment of atopic dermatitis and asthma, including in patients without a family history of psoriasis. In postmarketing reports, these cases resulted in partial or complete resolution of psoriasis with discontinuation of dupilumab, with or without use of supplemental treatment for psoriasis (topical or systemic). Those who continued dupilumab received supplemental treatment for psoriasis to improve associated symptoms. Advise patients to report new-onset psoriasis symptoms. If symptoms persist or worsen, consider dermatologic evaluation and/or discontinuation of DUPIXENT.
Arthralgia and Psoriatic Arthritis: Arthralgia has been reported with the use of DUPIXENT with some patients reporting gait disturbances or decreased mobility associated with joint symptoms; some cases resulted in hospitalization. Cases of new-onset psoriatic arthritis requiring systemic treatment have been reported with the use of DUPIXENT. Advise patients to report new-onset or worsening joint symptoms. If symptoms persist or worsen, consider rheumatological evaluation and/or discontinuation of DUPIXENT.
Parasitic (Helminth) Infections: It is unknown if DUPIXENT will influence the immune response against helminth infections. Treat patients with pre-existing helminth infections before initiating therapy with DUPIXENT. If patients become infected while receiving treatment with DUPIXENT and do not respond to anti-helminth treatment, discontinue treatment with DUPIXENT until the infection resolves. Helminth infections (5 cases of enterobiasis and 1 case of ascariasis) were reported in pediatric patients 6 to 11 years old in the pediatric asthma development program.
Vaccinations: Consider completing all age-appropriate vaccinations as recommended by current immunization guidelines prior to initiating DUPIXENT. Avoid use of live vaccines during treatment with DUPIXENT.
ADVERSE REACTIONS:
Most common adverse reactions are:
- Atopic Dermatitis (incidence ≥1%): injection site reactions, conjunctivitis, blepharitis, oral herpes, keratitis, eye pruritus, other herpes simplex virus infection, dry eye, and eosinophilia. The safety profile in pediatric patients through Week 16 was similar to that of adults with AD. In an open-label extension study, the long-term safety profile of DUPIXENT ± TCS in pediatric patients observed through Week 52 was consistent with that seen in adults with AD, with hand-foot-and-mouth disease and skin papilloma (incidence ≥2%) reported in patients 6 months to 5 years of age. These cases did not lead to study drug discontinuation.
- Asthma (incidence ≥1%): injection site reactions, oropharyngeal pain, and eosinophilia.
- Chronic Rhinosinusitis with Nasal Polyps (incidence ≥1% in adult patients): injection site reactions, eosinophilia, insomnia, toothache, gastritis, arthralgia, and conjunctivitis.
- Eosinophilic Esophagitis (incidence ≥2%): injection site reactions, upper respiratory tract infections, arthralgia, and herpes viral infections.
- Prurigo Nodularis (incidence ≥2%): nasopharyngitis, conjunctivitis, herpes infection, dizziness, myalgia, and diarrhea.
- Chronic Obstructive Pulmonary Disease (incidence ≥2%): viral infection, headache, nasopharyngitis, back pain, diarrhea, arthralgia, urinary tract infection, local administration reactions, rhinitis, eosinophilia, toothache, and gastritis.
- Chronic Spontaneous Urticaria (incidence ≥2%): injection site reactions.
USE IN SPECIFIC POPULATIONS
- Pregnancy: A pregnancy exposure registry monitors pregnancy outcomes in women exposed to DUPIXENT during pregnancy. To enroll or obtain information call 1‑877‑311‑8972 or go to https://mothertobaby.org/ongoing-study/dupixent/. Available data from case reports and case series with DUPIXENT use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Human IgG antibodies are known to cross the placental barrier; therefore, DUPIXENT may be transmitted from the mother to the developing fetus.
- Lactation: There are no data on the presence of DUPIXENT in human milk, the effects on the breastfed infant, or the effects on milk production. Maternal IgG is known to be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for DUPIXENT and any potential adverse effects on the breastfed child from DUPIXENT or from the underlying maternal condition.
Please see accompanying full Prescribing Information
INDICATIONS
Atopic Dermatitis: DUPIXENT is indicated for the treatment of adult and pediatric patients aged 6 months and older with moderate-to-severe atopic dermatitis (AD) whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. DUPIXENT can be used with or without topical corticosteroids.
Asthma: DUPIXENT is indicated as an add-on maintenance treatment of adult and pediatric patients aged 6 years and older with moderate-to-severe asthma characterized by an eosinophilic phenotype or with oral corticosteroid dependent asthma. Limitations of Use: DUPIXENT is not indicated for the relief of acute bronchospasm or status asthmaticus.
Chronic Rhinosinusitis with Nasal Polyps: DUPIXENT is indicated as an add-on maintenance treatment in adult and pediatric patients aged 12 years and older with inadequately controlled chronic rhinosinusitis with nasal polyps (CRSwNP).
Eosinophilic Esophagitis: DUPIXENT is indicated for the treatment of adult and pediatric patients aged 1 year and older, weighing at least 15 kg, with eosinophilic esophagitis (EoE).
Prurigo Nodularis: DUPIXENT is indicated for the treatment of adult patients with prurigo nodularis (PN).
Chronic Obstructive Pulmonary Disease: DUPIXENT is indicated as an add-on maintenance treatment of adult patients with inadequately controlled chronic obstructive pulmonary disease (COPD) and an eosinophilic phenotype. Limitations of Use: DUPIXENT is not indicated for the relief of acute bronchospasm.
Chronic Spontaneous Urticaria: DUPIXENT is indicated for the treatment of adult and pediatric patients aged 12 years and older with chronic spontaneous urticaria (CSU) who remain symptomatic despite H1 antihistamine treatment. Limitations of Use: DUPIXENT is not indicated for treatment of other forms of urticaria.
CONTRAINDICATION: DUPIXENT is contraindicated in patients with known hypersensitivity to dupilumab or any of its excipients.
WARNINGS AND PRECAUTIONS
Hypersensitivity: Hypersensitivity reactions, including anaphylaxis, serum sickness or serum sickness-like reactions, angioedema, generalized urticaria, rash, erythema nodosum, and erythema multiforme have been reported. If a clinically significant hypersensitivity reaction occurs, institute appropriate therapy and discontinue DUPIXENT.
Conjunctivitis and Keratitis: Conjunctivitis and keratitis occurred more frequently in AD subjects who received DUPIXENT versus placebo, with conjunctivitis being the most frequently reported eye disorder. Conjunctivitis also occurred more frequently in adult CRSwNP subjects, PN subjects, and COPD subjects who received DUPIXENT compared to those who received placebo. Conjunctivitis and keratitis have been reported with DUPIXENT in postmarketing settings, predominantly in AD patients. Some patients reported visual disturbances (e.g., blurred vision) associated with conjunctivitis or keratitis. Advise patients or their caregivers to report new-onset or worsening eye symptoms. Consider ophthalmological examination for patients who develop conjunctivitis that does not resolve following standard treatment or signs and symptoms suggestive of keratitis, as appropriate.
Eosinophilic Conditions: Patients being treated for asthma may present with clinical features of eosinophilic pneumonia or eosinophilic granulomatosis with polyangiitis (EGPA). These events may be associated with the reduction of oral corticosteroid therapy. Healthcare providers should be alert to vasculitic rash, worsening pulmonary symptoms, cardiac complications, kidney injury, and/or neuropathy presenting in their patients with eosinophilia. Cases of eosinophilic pneumonia were reported in adults who participated in the asthma development program and cases of EGPA have been reported with DUPIXENT in adults who participated in the asthma development program as well as in adults with co-morbid asthma in the CRSwNP development program. Advise patients to report signs of eosinophilic pneumonia and EGPA. Consider withholding DUPIXENT if eosinophilic pneumonia or EGPA are suspected.
Acute Symptoms of Asthma or Chronic Obstructive Pulmonary Disease or Acute Deteriorating Disease: Do not use DUPIXENT to treat acute symptoms or acute exacerbations of asthma or COPD, acute bronchospasm, or status asthmaticus. Patients should seek medical advice if their asthma or COPD remains uncontrolled or worsens after initiation of DUPIXENT.
Risk Associated with Abrupt Reduction of Corticosteroid Dosage: Do not discontinue systemic, topical, or inhaled corticosteroids abruptly upon initiation of DUPIXENT. Reductions in corticosteroid dose, if appropriate, should be gradual and performed under the direct supervision of a healthcare provider. Reduction in corticosteroid dose may be associated with systemic withdrawal symptoms and/or unmask conditions previously suppressed by systemic corticosteroid therapy.
Patients with Co-morbid Asthma: Advise patients with co-morbid asthma not to adjust or stop their asthma treatments without consultation with their physicians.
Psoriasis: Cases of new-onset psoriasis have been reported with the use of DUPIXENT for the treatment of atopic dermatitis and asthma, including in patients without a family history of psoriasis. In postmarketing reports, these cases resulted in partial or complete resolution of psoriasis with discontinuation of dupilumab, with or without use of supplemental treatment for psoriasis (topical or systemic). Those who continued dupilumab received supplemental treatment for psoriasis to improve associated symptoms. Advise patients to report new-onset psoriasis symptoms. If symptoms persist or worsen, consider dermatologic evaluation and/or discontinuation of DUPIXENT.
Arthralgia and Psoriatic Arthritis: Arthralgia has been reported with the use of DUPIXENT with some patients reporting gait disturbances or decreased mobility associated with joint symptoms; some cases resulted in hospitalization. Cases of new-onset psoriatic arthritis requiring systemic treatment have been reported with the use of DUPIXENT. Advise patients to report new-onset or worsening joint symptoms. If symptoms persist or worsen, consider rheumatological evaluation and/or discontinuation of DUPIXENT.
Parasitic (Helminth) Infections: It is unknown if DUPIXENT will influence the immune response against helminth infections. Treat patients with pre-existing helminth infections before initiating therapy with DUPIXENT. If patients become infected while receiving treatment with DUPIXENT and do not respond to anti-helminth treatment, discontinue treatment with DUPIXENT until the infection resolves. Helminth infections (5 cases of enterobiasis and 1 case of ascariasis) were reported in pediatric patients 6 to 11 years old in the pediatric asthma development program.
Vaccinations: Consider completing all age-appropriate vaccinations as recommended by current immunization guidelines prior to initiating DUPIXENT. Avoid use of live vaccines during treatment with DUPIXENT.
ADVERSE REACTIONS:
Most common adverse reactions are:
- Atopic Dermatitis (incidence ≥1%): injection site reactions, conjunctivitis, blepharitis, oral herpes, keratitis, eye pruritus, other herpes simplex virus infection, dry eye, and eosinophilia. The safety profile in pediatric patients through Week 16 was similar to that of adults with AD. In an open-label extension study, the long-term safety profile of DUPIXENT ± TCS in pediatric patients observed through Week 52 was consistent with that seen in adults with AD, with hand-foot-and-mouth disease and skin papilloma (incidence ≥2%) reported in patients 6 months to 5 years of age. These cases did not lead to study drug discontinuation.
- Asthma (incidence ≥1%): injection site reactions, oropharyngeal pain, and eosinophilia.
- Chronic Rhinosinusitis with Nasal Polyps (incidence ≥1% in adult patients): injection site reactions, eosinophilia, insomnia, toothache, gastritis, arthralgia, and conjunctivitis.
- Eosinophilic Esophagitis (incidence ≥2%): injection site reactions, upper respiratory tract infections, arthralgia, and herpes viral infections.
- Prurigo Nodularis (incidence ≥2%): nasopharyngitis, conjunctivitis, herpes infection, dizziness, myalgia, and diarrhea.
- Chronic Obstructive Pulmonary Disease (incidence ≥2%): viral infection, headache, nasopharyngitis, back pain, diarrhea, arthralgia, urinary tract infection, local administration reactions, rhinitis, eosinophilia, toothache, and gastritis.
- Chronic Spontaneous Urticaria (incidence ≥2%): injection site reactions.
USE IN SPECIFIC POPULATIONS
- Pregnancy: A pregnancy exposure registry monitors pregnancy outcomes in women exposed to DUPIXENT during pregnancy. To enroll or obtain information call 1‑877‑311‑8972 or go to https://mothertobaby.org/ongoing-study/dupixent/. Available data from case reports and case series with DUPIXENT use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Human IgG antibodies are known to cross the placental barrier; therefore, DUPIXENT may be transmitted from the mother to the developing fetus.
- Lactation: There are no data on the presence of DUPIXENT in human milk, the effects on the breastfed infant, or the effects on milk production. Maternal IgG is known to be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for DUPIXENT and any potential adverse effects on the breastfed child from DUPIXENT or from the underlying maternal condition.
Please see accompanying full Prescribing Information
INDICATIONS
Atopic Dermatitis: DUPIXENT is indicated for the treatment of adult and pediatric patients aged 6 months and older with moderate-to-severe atopic dermatitis (AD) whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. DUPIXENT can be used with or without topical corticosteroids.
Asthma: DUPIXENT is indicated as an add-on maintenance treatment of adult and pediatric patients aged 6 years and older with moderate-to-severe asthma characterized by an eosinophilic phenotype or with oral corticosteroid dependent asthma. Limitations of Use: DUPIXENT is not indicated for the relief of acute bronchospasm or status asthmaticus.
Chronic Rhinosinusitis with Nasal Polyps: DUPIXENT is indicated as an add-on maintenance treatment in adult and pediatric patients aged 12 years and older with inadequately controlled chronic rhinosinusitis with nasal polyps (CRSwNP).
Eosinophilic Esophagitis: DUPIXENT is indicated for the treatment of adult and pediatric patients aged 1 year and older, weighing at least 15 kg, with eosinophilic esophagitis (EoE).
Prurigo Nodularis: DUPIXENT is indicated for the treatment of adult patients with prurigo nodularis (PN).
Chronic Obstructive Pulmonary Disease: DUPIXENT is indicated as an add-on maintenance treatment of adult patients with inadequately controlled chronic obstructive pulmonary disease (COPD) and an eosinophilic phenotype. Limitations of Use: DUPIXENT is not indicated for the relief of acute bronchospasm.
Chronic Spontaneous Urticaria: DUPIXENT is indicated for the treatment of adult and pediatric patients aged 12 years and older with chronic spontaneous urticaria (CSU) who remain symptomatic despite H1 antihistamine treatment. Limitations of Use: DUPIXENT is not indicated for treatment of other forms of urticaria.
CONTRAINDICATION: DUPIXENT is contraindicated in patients with known hypersensitivity to dupilumab or any of its excipients.
WARNINGS AND PRECAUTIONS
Hypersensitivity: Hypersensitivity reactions, including anaphylaxis, serum sickness or serum sickness-like reactions, angioedema, generalized urticaria, rash, erythema nodosum, and erythema multiforme have been reported. If a clinically significant hypersensitivity reaction occurs, institute appropriate therapy and discontinue DUPIXENT.
Conjunctivitis and Keratitis: Conjunctivitis and keratitis occurred more frequently in AD subjects who received DUPIXENT versus placebo, with conjunctivitis being the most frequently reported eye disorder. Conjunctivitis also occurred more frequently in adult CRSwNP subjects, PN subjects, and COPD subjects who received DUPIXENT compared to those who received placebo. Conjunctivitis and keratitis have been reported with DUPIXENT in postmarketing settings, predominantly in AD patients. Some patients reported visual disturbances (e.g., blurred vision) associated with conjunctivitis or keratitis. Advise patients or their caregivers to report new-onset or worsening eye symptoms. Consider ophthalmological examination for patients who develop conjunctivitis that does not resolve following standard treatment or signs and symptoms suggestive of keratitis, as appropriate.
Eosinophilic Conditions: Patients being treated for asthma may present with clinical features of eosinophilic pneumonia or eosinophilic granulomatosis with polyangiitis (EGPA). These events may be associated with the reduction of oral corticosteroid therapy. Healthcare providers should be alert to vasculitic rash, worsening pulmonary symptoms, cardiac complications, kidney injury, and/or neuropathy presenting in their patients with eosinophilia. Cases of eosinophilic pneumonia were reported in adults who participated in the asthma development program and cases of EGPA have been reported with DUPIXENT in adults who participated in the asthma development program as well as in adults with co-morbid asthma in the CRSwNP development program. Advise patients to report signs of eosinophilic pneumonia and EGPA. Consider withholding DUPIXENT if eosinophilic pneumonia or EGPA are suspected.
Acute Symptoms of Asthma or Chronic Obstructive Pulmonary Disease or Acute Deteriorating Disease: Do not use DUPIXENT to treat acute symptoms or acute exacerbations of asthma or COPD, acute bronchospasm, or status asthmaticus. Patients should seek medical advice if their asthma or COPD remains uncontrolled or worsens after initiation of DUPIXENT.
Risk Associated with Abrupt Reduction of Corticosteroid Dosage: Do not discontinue systemic, topical, or inhaled corticosteroids abruptly upon initiation of DUPIXENT. Reductions in corticosteroid dose, if appropriate, should be gradual and performed under the direct supervision of a healthcare provider. Reduction in corticosteroid dose may be associated with systemic withdrawal symptoms and/or unmask conditions previously suppressed by systemic corticosteroid therapy.
Patients with Co-morbid Asthma: Advise patients with co-morbid asthma not to adjust or stop their asthma treatments without consultation with their physicians.
Psoriasis: Cases of new-onset psoriasis have been reported with the use of DUPIXENT for the treatment of atopic dermatitis and asthma, including in patients without a family history of psoriasis. In postmarketing reports, these cases resulted in partial or complete resolution of psoriasis with discontinuation of dupilumab, with or without use of supplemental treatment for psoriasis (topical or systemic). Those who continued dupilumab received supplemental treatment for psoriasis to improve associated symptoms. Advise patients to report new-onset psoriasis symptoms. If symptoms persist or worsen, consider dermatologic evaluation and/or discontinuation of DUPIXENT.
Arthralgia and Psoriatic Arthritis: Arthralgia has been reported with the use of DUPIXENT with some patients reporting gait disturbances or decreased mobility associated with joint symptoms; some cases resulted in hospitalization. Cases of new-onset psoriatic arthritis requiring systemic treatment have been reported with the use of DUPIXENT. Advise patients to report new-onset or worsening joint symptoms. If symptoms persist or worsen, consider rheumatological evaluation and/or discontinuation of DUPIXENT.
Parasitic (Helminth) Infections: It is unknown if DUPIXENT will influence the immune response against helminth infections. Treat patients with pre-existing helminth infections before initiating therapy with DUPIXENT. If patients become infected while receiving treatment with DUPIXENT and do not respond to anti-helminth treatment, discontinue treatment with DUPIXENT until the infection resolves. Helminth infections (5 cases of enterobiasis and 1 case of ascariasis) were reported in pediatric patients 6 to 11 years old in the pediatric asthma development program.
Vaccinations: Consider completing all age-appropriate vaccinations as recommended by current immunization guidelines prior to initiating DUPIXENT. Avoid use of live vaccines during treatment with DUPIXENT.
ADVERSE REACTIONS:
Most common adverse reactions are:
- Atopic Dermatitis (incidence ≥1%): injection site reactions, conjunctivitis, blepharitis, oral herpes, keratitis, eye pruritus, other herpes simplex virus infection, dry eye, and eosinophilia. The safety profile in pediatric patients through Week 16 was similar to that of adults with AD. In an open-label extension study, the long-term safety profile of DUPIXENT ± TCS in pediatric patients observed through Week 52 was consistent with that seen in adults with AD, with hand-foot-and-mouth disease and skin papilloma (incidence ≥2%) reported in patients 6 months to 5 years of age. These cases did not lead to study drug discontinuation.
- Asthma (incidence ≥1%): injection site reactions, oropharyngeal pain, and eosinophilia.
- Chronic Rhinosinusitis with Nasal Polyps (incidence ≥1% in adult patients): injection site reactions, eosinophilia, insomnia, toothache, gastritis, arthralgia, and conjunctivitis.
- Eosinophilic Esophagitis (incidence ≥2%): injection site reactions, upper respiratory tract infections, arthralgia, and herpes viral infections.
- Prurigo Nodularis (incidence ≥2%): nasopharyngitis, conjunctivitis, herpes infection, dizziness, myalgia, and diarrhea.
- Chronic Obstructive Pulmonary Disease (incidence ≥2%): viral infection, headache, nasopharyngitis, back pain, diarrhea, arthralgia, urinary tract infection, local administration reactions, rhinitis, eosinophilia, toothache, and gastritis.
- Chronic Spontaneous Urticaria (incidence ≥2%): injection site reactions.
USE IN SPECIFIC POPULATIONS
- Pregnancy: A pregnancy exposure registry monitors pregnancy outcomes in women exposed to DUPIXENT during pregnancy. To enroll or obtain information call 1‑877‑311‑8972 or go to https://mothertobaby.org/ongoing-study/dupixent/. Available data from case reports and case series with DUPIXENT use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Human IgG antibodies are known to cross the placental barrier; therefore, DUPIXENT may be transmitted from the mother to the developing fetus.
- Lactation: There are no data on the presence of DUPIXENT in human milk, the effects on the breastfed infant, or the effects on milk production. Maternal IgG is known to be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for DUPIXENT and any potential adverse effects on the breastfed child from DUPIXENT or from the underlying maternal condition.
Please see accompanying full Prescribing Information
This site is intended for US payers, formulary committees, or other similar entities for purposes of population-based drug selection, coverage, and/or reimbursement decision-making, pursuant to FD&C Act Section 502(a).
© 2025 Sanofi and Regeneron Pharmaceuticals, Inc. All Rights Reserved.
DUPIXENT® and DUPIXENT MyWay® are registered trademarks of Sanofi Biotechnology.
Sanofi US is hosting this website on behalf of Sanofi and Regeneron Pharmaceuticals, Inc. Sanofi and Regeneron are industry partners, who are committed to handling personal data in ways that respect your privacy. Both companies may independently process your personal data to manage patient support programs and product marketing campaigns. Please refer to Regeneron’s Privacy Notice and Sanofi’s Privacy Policy and Cookies Policy for more information regarding processing of your personal data.