Multiple sclerosis (MS)
New solutions are needed in the MS market
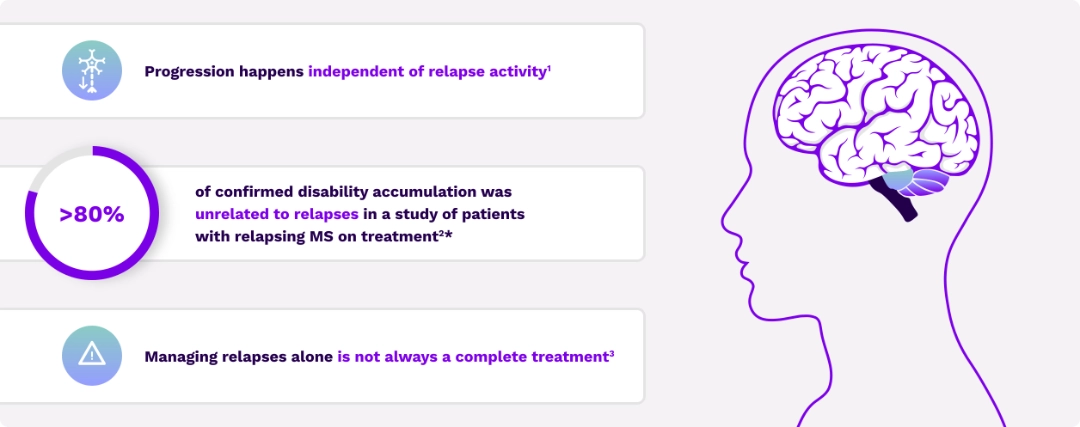
Understanding of the biology and importance of disability accumulation has evolved
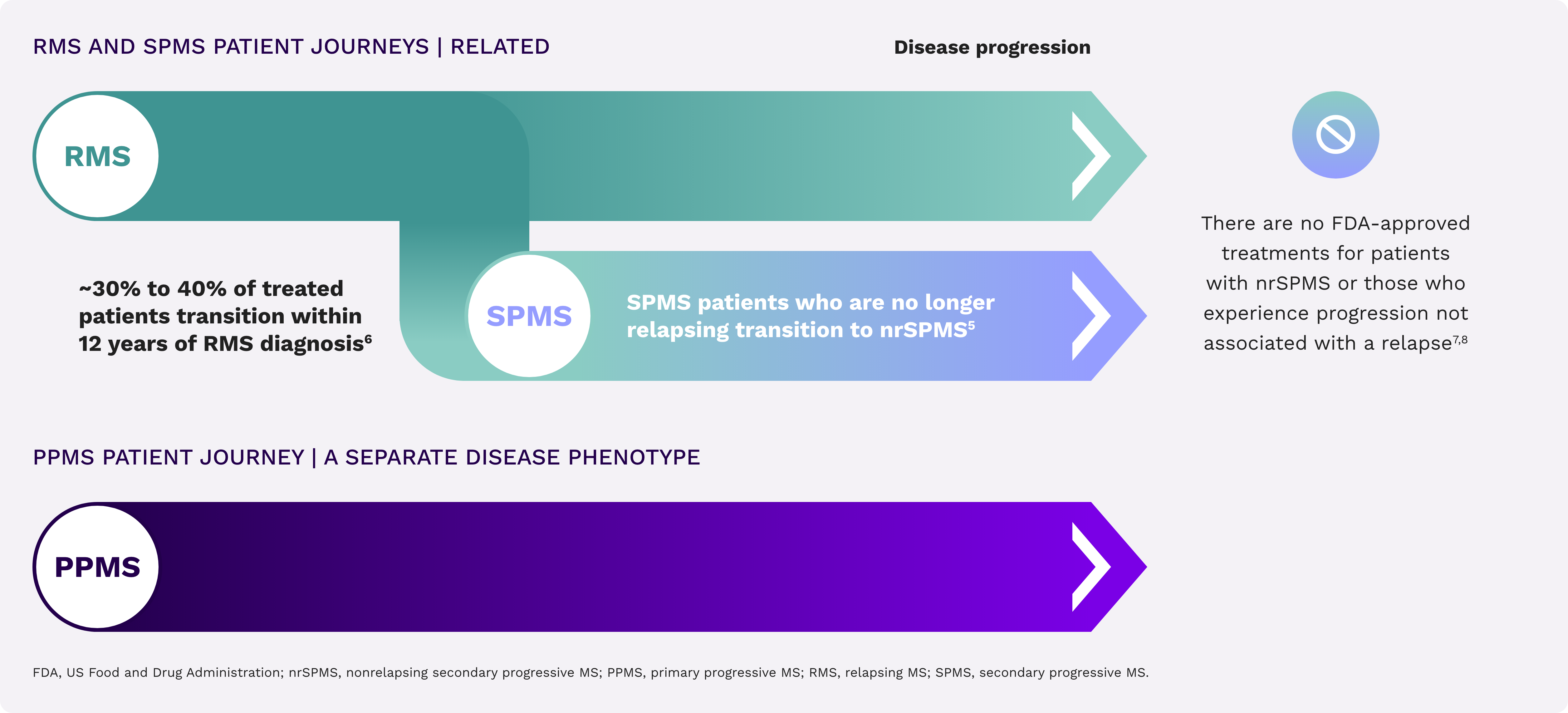
Each type of MS has a unique path toward irreversible disability accumulation4,5
Disability accumulation originates from 2 different processes3
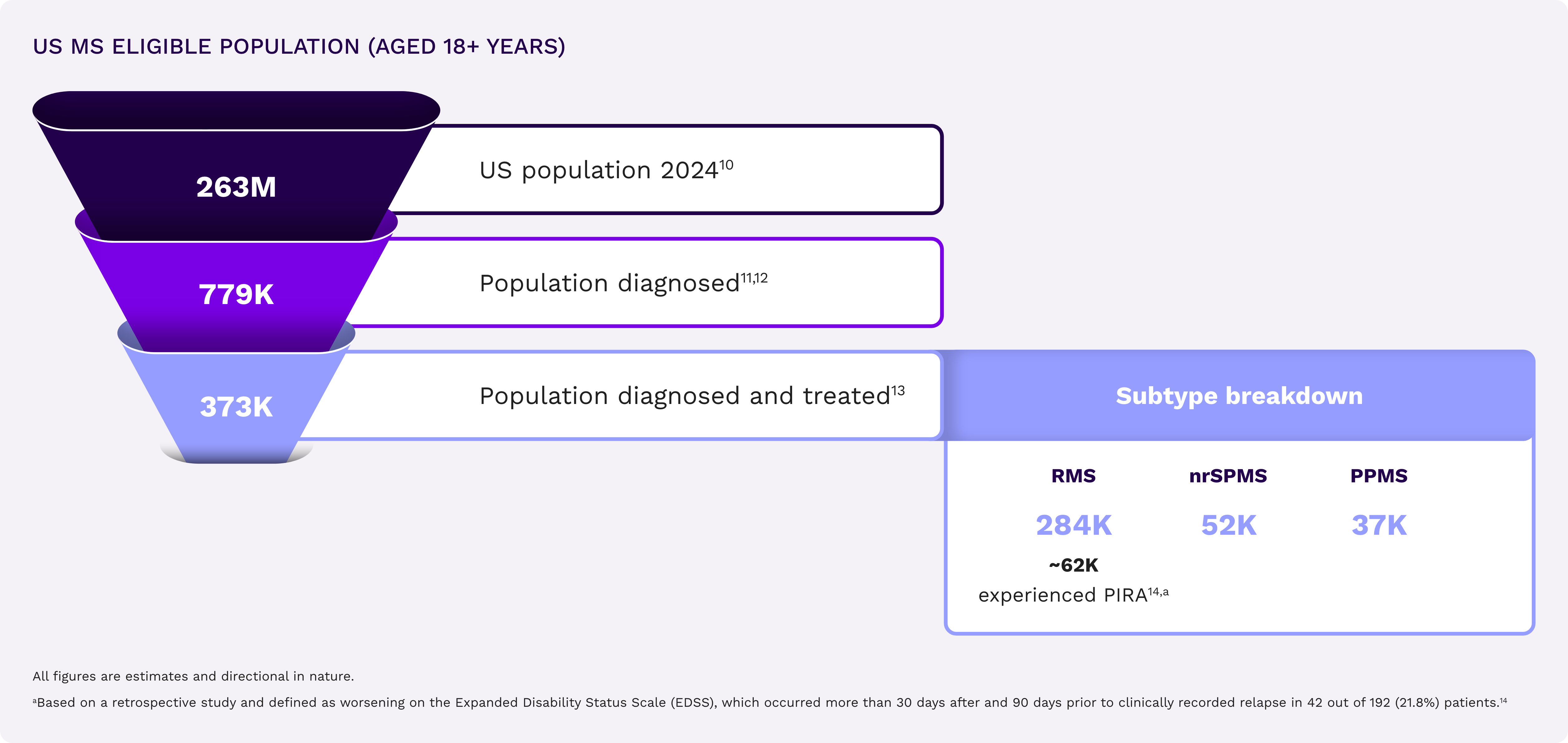
Across the MS spectrum, unmet needs remain for your members
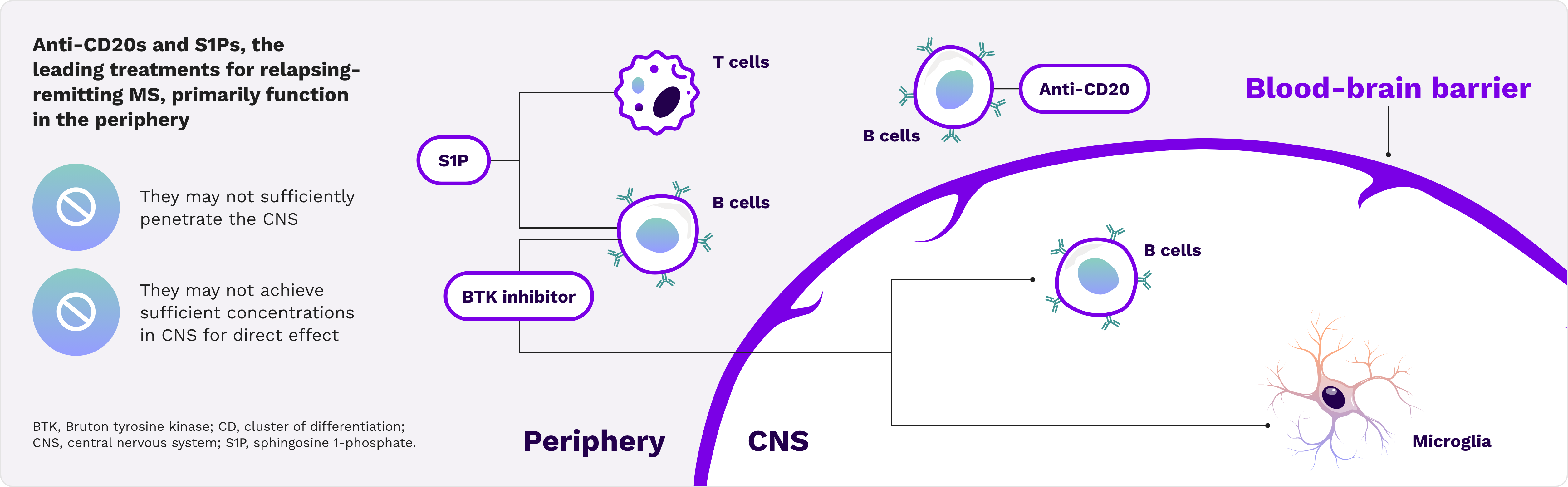
Current treatments may not completely impact drivers of disability accumulation15-20
Exclusive, near-term pipeline information for payers only
Designed specifically to support your early formulary decisions
Unlock access to our featured pipeline product investigating MS*
*This content is exclusive to population-based decision makers. If your email is auto-verified, you will be granted immediate access. If you are not auto-verified, registration is required.
References: 1. Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145(9):3147–3161. doi:10.1093/brain/awac016 2. Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77(9):1132–1140. doi:10.1001/jamaneurol.2020.1568 3. Giovannoni G, Popescu V, Wuerfel J, et al. Smouldering multiple sclerosis: the ‘real MS’. Ther Adv Neurol Disord. 2022;15:17562864211066751. doi:10.1177/17562864211066751 4. Tur C, Carbonell-Mirabent P, Cobo-Calvo Á, et al. Association of early progression independent of relapse activity with long-term disability after a first demyelinating event in multiple sclerosis. JAMA Neurol. 2023;80(2):151–160. doi:10.1001/jamaneurol.2022.4655 5. Secondary progressive multiple sclerosis (SPMS). National Multiple Sclerosis Society. Accessed June 13, 2025. https://www.nationalmssociety.org/understanding-ms/what-is-ms/types-of-ms/progressive-ms 6. Coret F, Pérez-Miralles FC, Gascón F, et al. Onset of secondary progressive multiple sclerosis is not influenced by current relapsing multiple sclerosis therapies. Mult Scler J Exp Transl Clin. 2018;4(2):2055217318783347. doi:10.1177/2055217318783347 7. Manjunatha RT, Habib S, Sangaraju SL, Yepez D, Grandes XA. Multiple sclerosis: therapeutic strategies on the horizon. Cureus. 2022;14(5):e24895. doi:10.7759/cureus.24895 8. Disease modification. National Multiple Sclerosis Society. Accessed June 13, 2025. https://www.nationalmssociety.org/for-professionals/for-healthcare-professionals/managing-and-treating-ms/disease-modification 9. Scalfari A, Traboulsee A, Oh J, et al. Smouldering-associated worsening in multiple sclerosis: an international consensus statement on definition, biology, clinical implications, and future directions. Ann Neurol. 2024;96(5):826–845. doi:10.1002/ana.27034 10. S0101: Age and sex 2023 American Community Survey 1-year estimates. US Census Bureau. Accessed June 13, 2025. https://data.census.gov/table/ACSST1Y2023.S0101?q=United States 11. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029–e1040. doi:10.1212/WNL.0000000000007035 12. Data on file. Sanofi US. 13. Zhang Y, Salter A, Jin S, et al. Disease-modifying therapy prescription patterns in people with multiple sclerosis by age. Ther Adv Neurol Disord. 2021;14:17562864211006499. doi:10.1177/17562864211006499 14. Fuchs TA, Schoonheim MM, Zivadinov R, et al. Cognitive progression independent of relapse in multiple sclerosis. Mult Scler. 2024;30(11–12):1468–1478. doi:10.1177/13524585241256508 15. Correale J, Halfon MJ, Jack D, Rubinstein A, Villa A. Acting centrally or peripherally: a renewed interest in the central nervous system penetration of disease-modifying drugs in multiple sclerosis. Mult Scler Relat Disord. 2021;56:103264. doi:10.1016/j.msard.2021.103264 16. Oh J, Bar-Or A. Emerging therapies to target CNS pathophysiology in multiple sclerosis. Nat Rev Neurol. 2022;18(8):466–475. doi:10.1038/s41582-022-00675-0 17. Turner TJ, Brun P, Gruber RC, Ofengeim D. Comparative CNS pharmacology of the Bruton’s tyrosine kinase (BTK) inhibitor tolebrutinib versus other BTK inhibitor candidates for treating multiple sclerosis. Drugs R D. 2024;24(2):263–274. doi:10.1007/s40268-024-00468-4 18. Galesaria A, Häusler D, Weber MS. Microglia: the missing link to decipher and therapeutically control MS progression? Int J Mol Sci. 2021;22(7):3461. doi:10.3390/ijms22073461 19. Siden M, Rissanen E, Tuisku J, et al. Evaluation of the effect of fingolimod treatment on microglial activation using serial PET imaging in multiple sclerosis. J Nucl Med. 2017;58(10):1646–1651. doi:10.2967/jnumed.116.183020 20. Yang JH, Rempe T, Whitmire N, Dunn-Pirio A, Graves JS. Therapeutic advances in multiple sclerosis. Front Neurol. 2022;13:824926. doi:10.3389/fneur.2022.824926
This site is intended for US payers only.
© 2025 Sanofi. All rights reserved.