Type 1 diabetes: A
progressive autoimmune
disease with lifelong
consequences
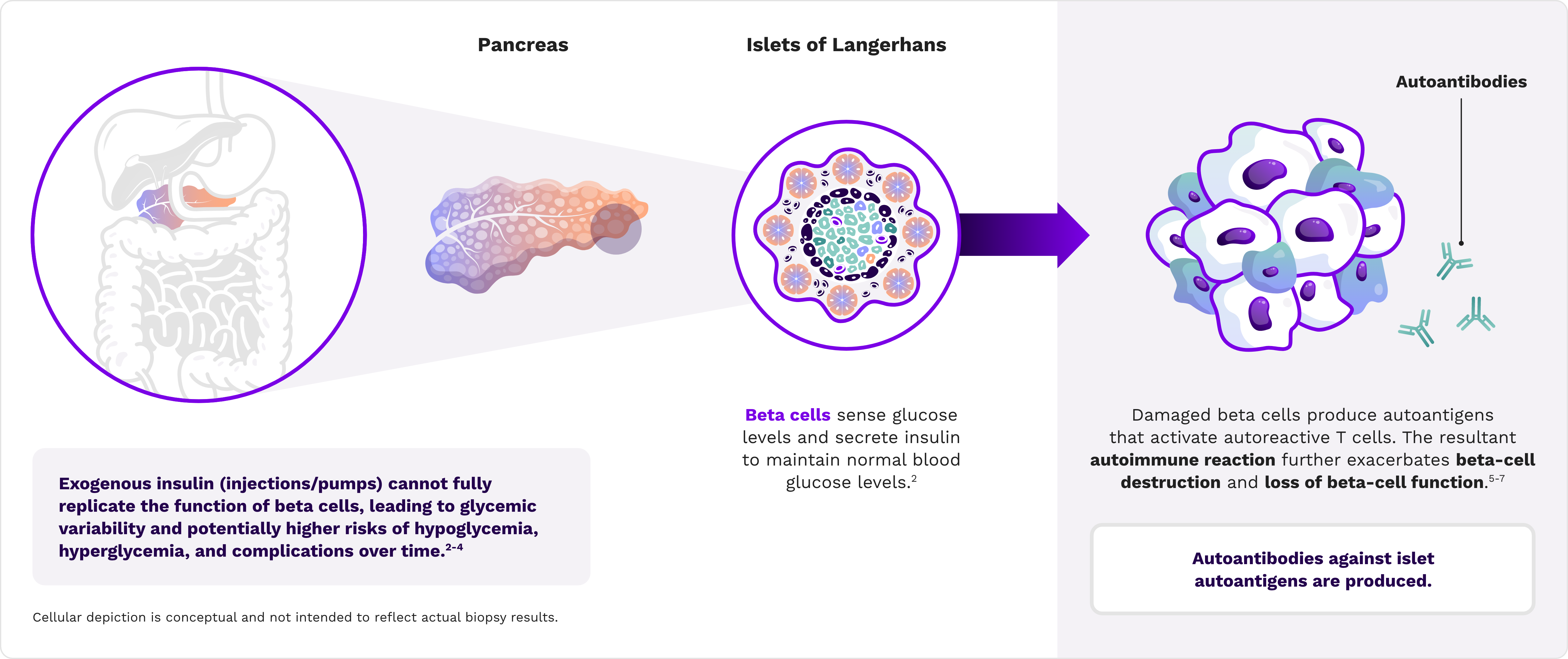
Tight glycemic regulation requires endogenous insulin production by pancreatic beta cells1,2
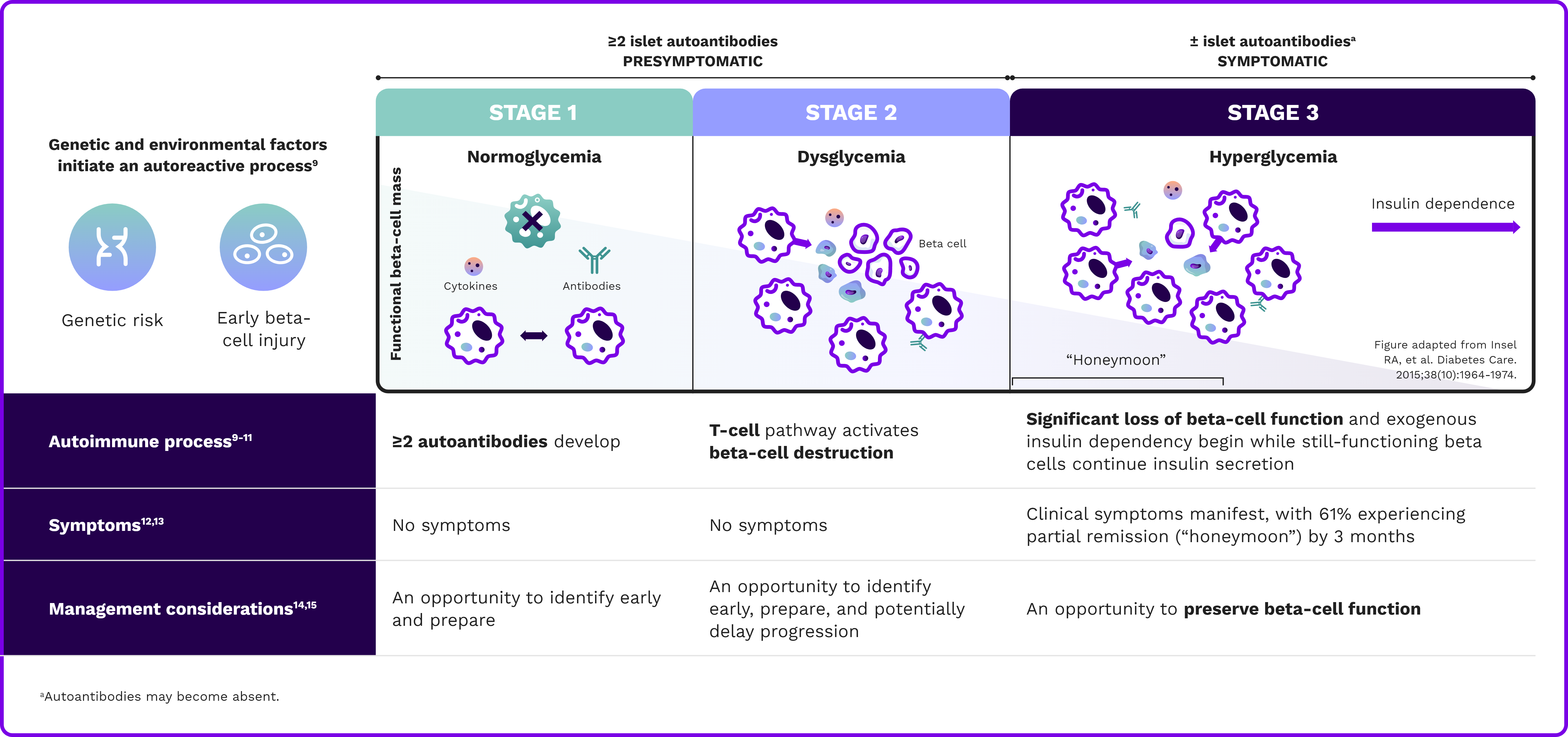
Type 1 diabetes is a progressive autoimmune disease characterized by the destruction of insulin-producing pancreatic beta cells across 3 stages8-10
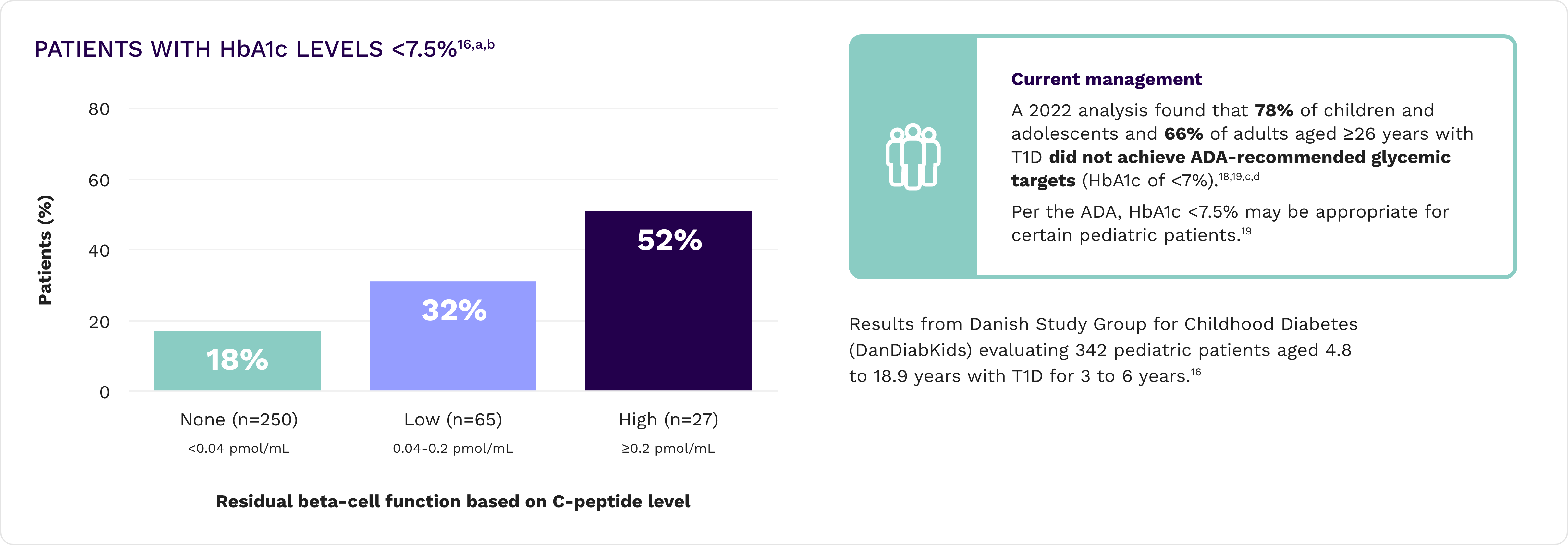
Even minimal residual beta-cell function can impact clinical and economic outcomes
Observed clinical impact
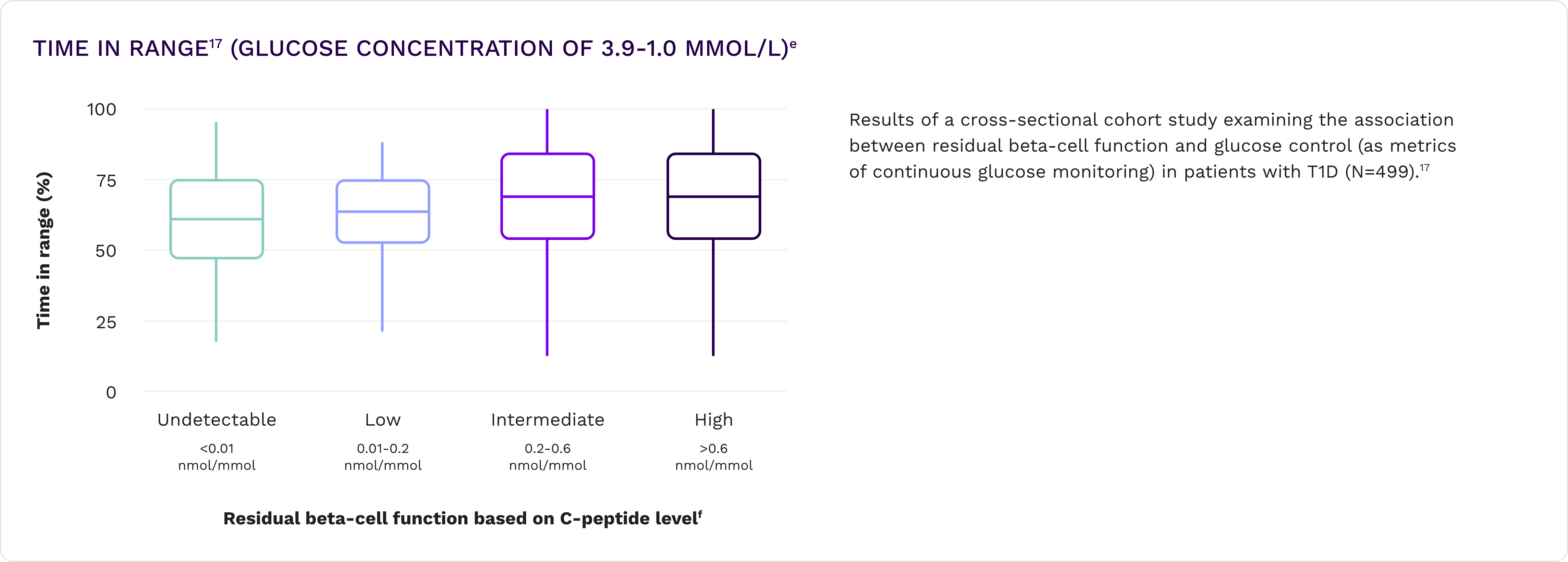
Lower HbA1c and improved time in range16,17
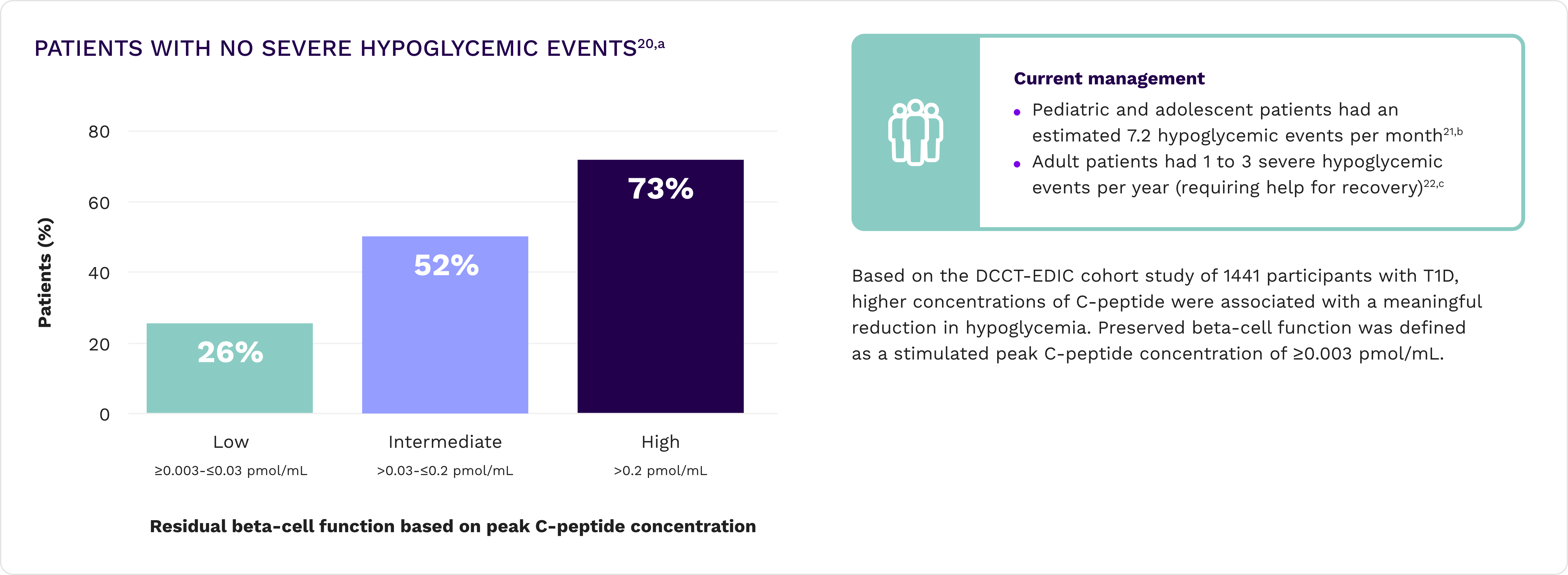
Fewer hypoglycemic events20
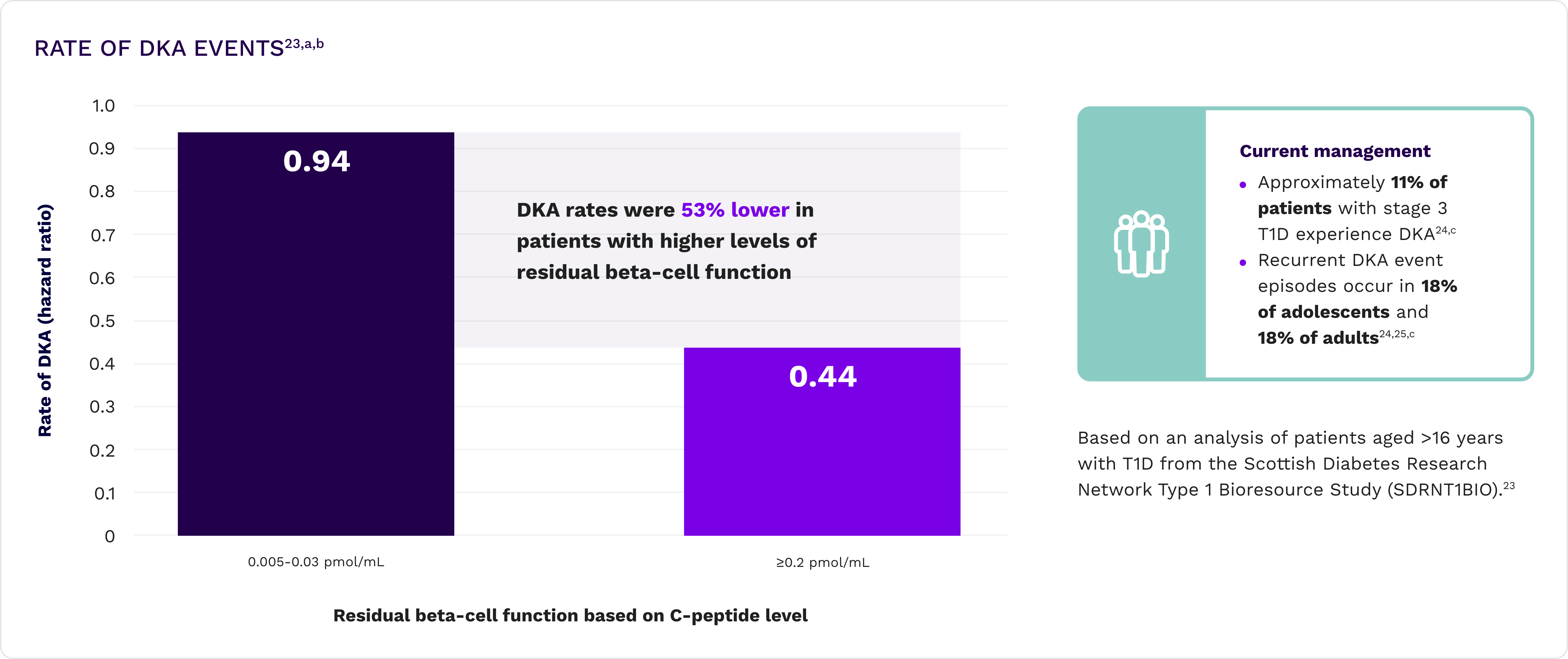
Reduced DKA events23

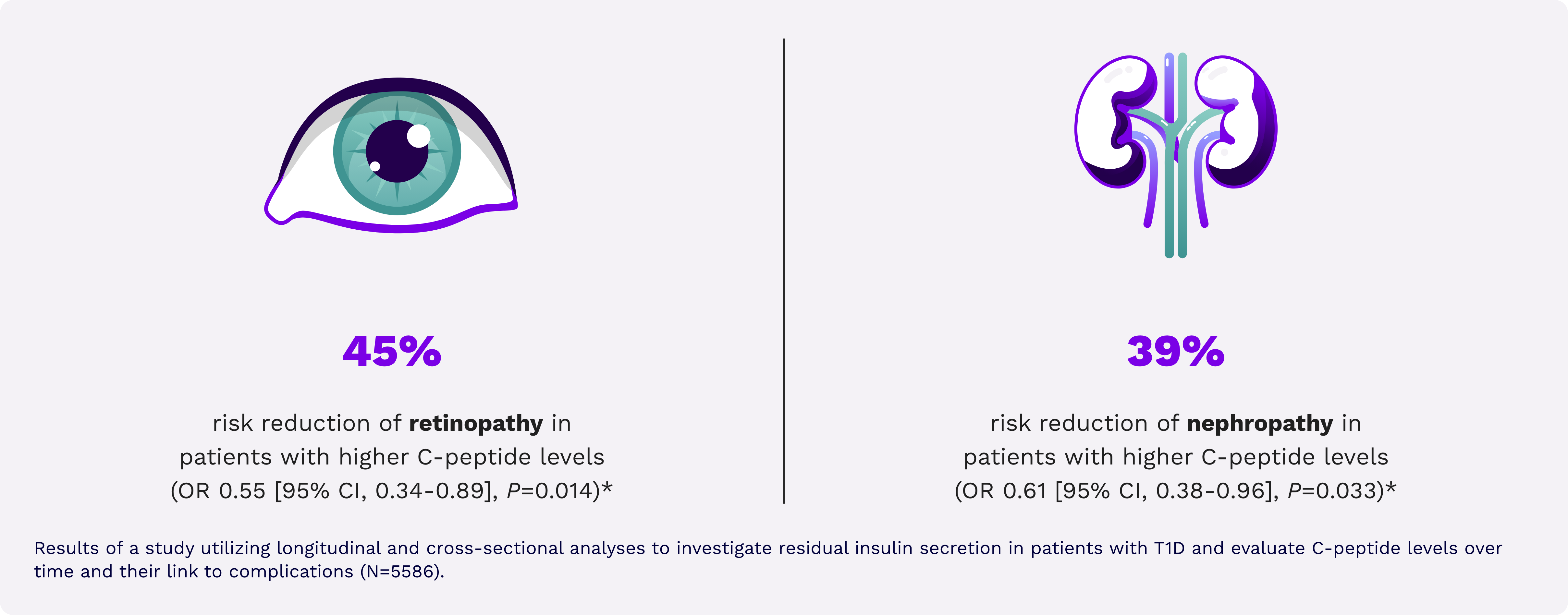
Reduced risk of microvascular complications26
Observed economic impact
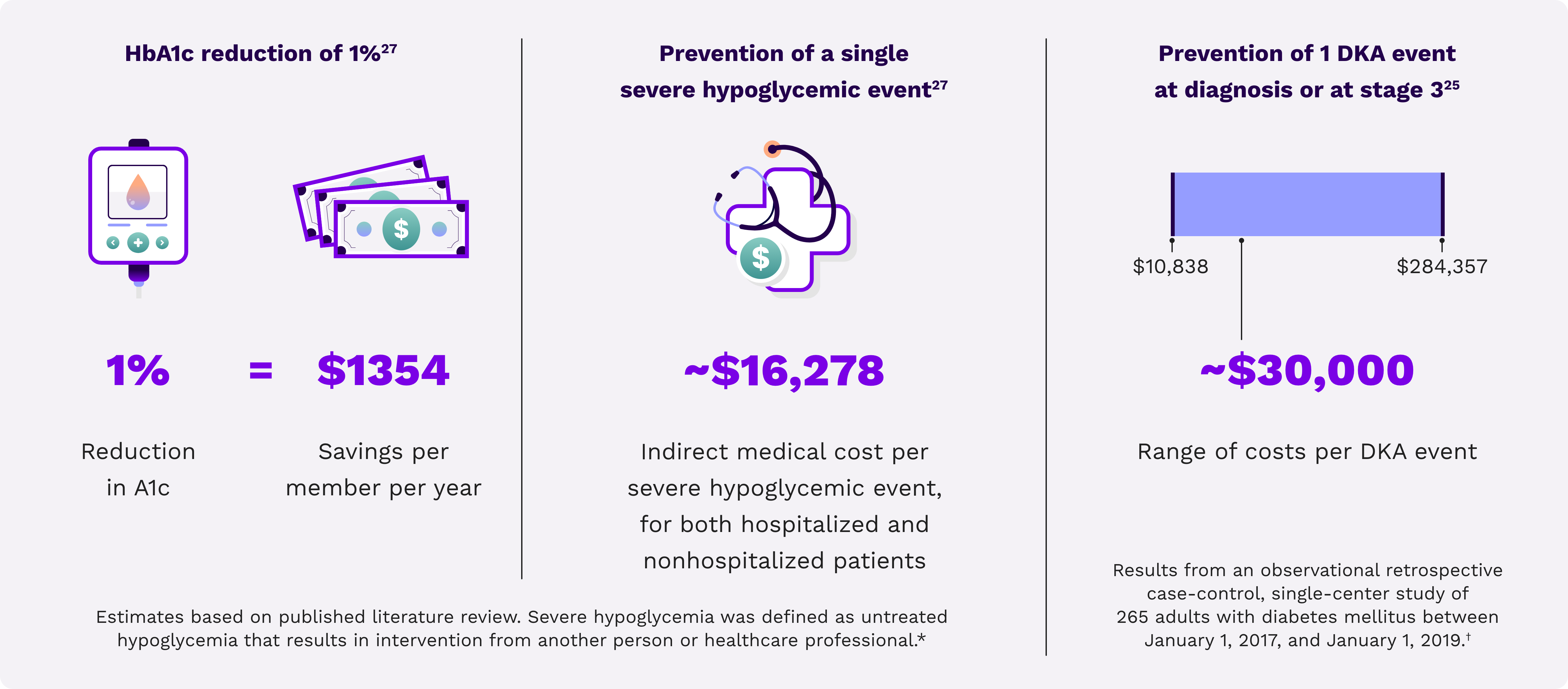
Cost savings associated with improved HbA1c27
Reduction of DKA-related costs25
Lower hypoglycemia-related costs27
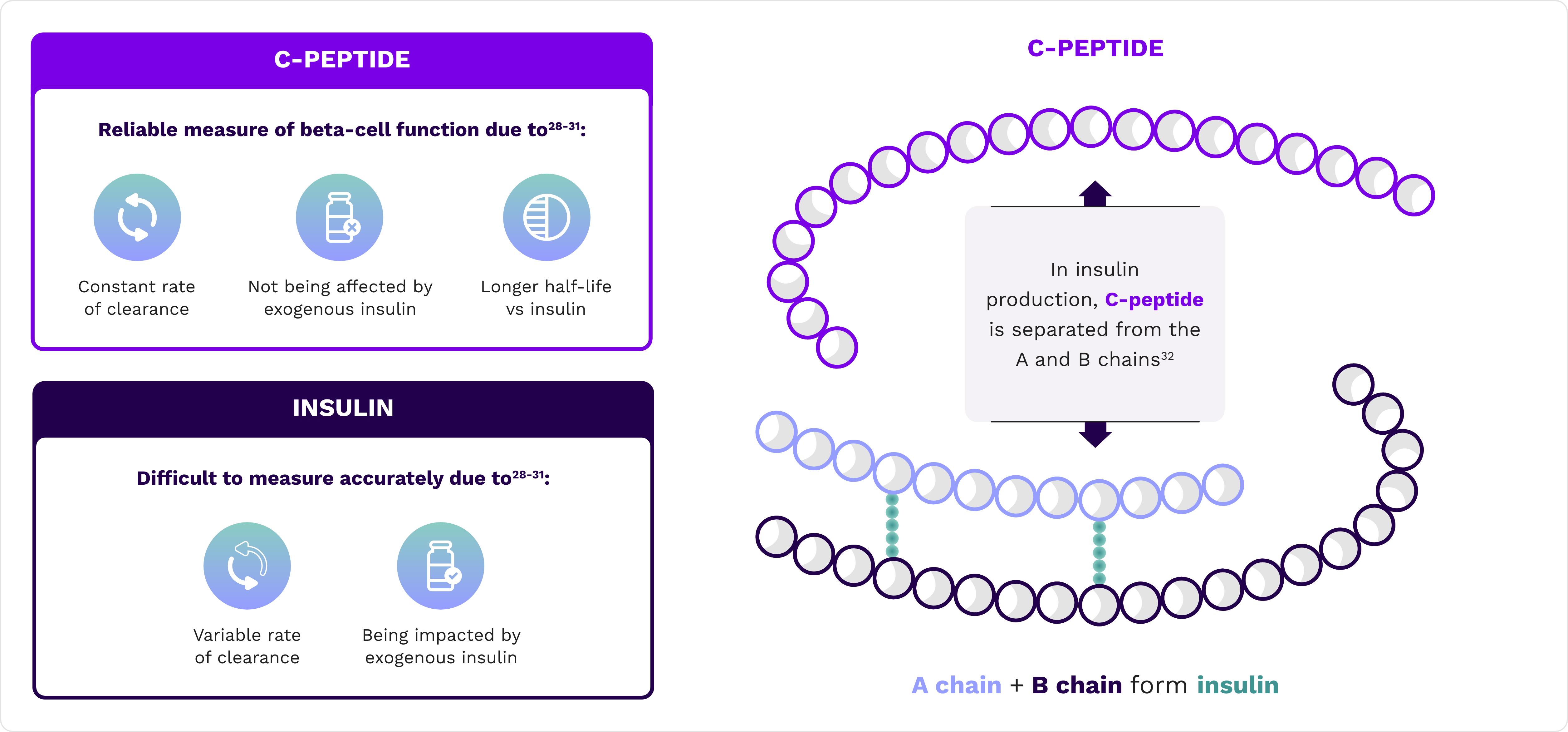
C-peptide is used to assess pancreatic beta-cell function
C-peptide is a byproduct of insulin biogenesis and is released by beta cells at a 1:1 ratio with insulin28
ADA, American Diabetes Association; DKA, diabetic ketoacidosis; FDA, US Food and Drug Administration; HbA1c, hemoglobin A1c; T1D, type 1 diabetes.
References: 1. Röder PV, Wu B, Liu Y, Han W. Pancreatic regulation of glucose homeostasis. Exp Mol Med. 2016;48(3):e219. doi:10.1038/emm.2016.6 2. Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464(7293):1293-1300. doi:10.1038/nature08933 3. Holt RIG, DeVries JH, Hess-Fischl A, et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2021;64(12):2609-2652. doi:10.1007/s00125-021-05568-3 4. Kaestner KH, Campbell-Thompson M, Dor Y, et al. What is a β cell? – Chapter I in the Human Islet Research Network (HIRN) review series. Mol Metab. 2021;53:101323. doi:10.1016/j.molmet.2021.101323 5. Xie Z, Chang C, Zhou Z. Molecular mechanisms in autoimmune type 1 diabetes: a critical review. Clin Rev Allergy Immunol. 2014;47(2):174-192. doi:10.1007/s12016-014-8422-2 6. Yoon JW, Jun HS. Autoimmune destruction of pancreatic beta cells. Am J Ther. 2005;12(6):580-591. doi:10.1097/01.mjt.0000178767.67857.63 7. Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol. 2020;16(7):349-362. doi:10.1038/s41574-020-0355-7 8. Toren E, Burnette KS, Banerjee RR, Hunter CS, Tse HM. Partners in crime: beta-cells and autoimmune responses complicit in type 1 diabetes pathogenesis. Front Immunol. 2021;12:756548. doi:10.3389/fimmu.2021.756548 9. Skyler JS, Bakris GL, Bonifacio E, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. 2017;66(2):241-255. doi:10.2337/db16-0806 10. Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38(10):1964-1974. doi:10.2337/dc15-1419 11. DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391(10138):2449-2462. doi:10.1016/S0140-6736(18)31320-5 12. American Diabetes Association Professional Practice Committee. Diagnosis and classification of diabetes: standards of care in diabetes-2025. Diabetes Care. 2025;48(suppl 1):S27-S49. doi:10.2337/dc25-S002 13. Mortensen HB, Hougaard P, Swift P, et al. New definition for the partial remission period in children and adolescents with type 1 diabetes. Diabetes Care. 2009;32(8):1384-1390. doi:10.2337/dc08-1987 14. Haller MJ, Bell KJ, Besser REJ, et al. ISPAD Clinical Practice Consensus Guidelines 2024: screening, staging, and strategies to preserve beta-cell function in children and adolescents with type 1 diabetes. Horm Res Paediatr. 2024;97(6):529-545. doi:10.1159/000543035 15. Sundheim B, Hirani K, Blaschke M, Lemos JRN, Mittal R. Pre-type 1 diabetes in adolescents and teens: screening, nutritional interventions, beta-cell preservation, and psychosocial impacts. J Clin Med. 2025;14(2):383. doi:10.3390/jcm14020383 16. Sørensen JS, Johannesen J, Pociot F, et al. Residual β-cell function 3-6 years after onset of type 1 diabetes reduces risk of severe hypoglycemia in children and adolescents. Diabetes Care. 2013;36(11):3454-3459. doi:10.2337/dc13-0418 17. Fuhri Snethlage CM, McDonald TJ, Oram RD, et al. Residual β-cell function is associated with longer time in range in individuals with type 1 diabetes. Diabetes Care. 2024;47(7):1114-1121. doi:10.2337/dc23-0776 18. Ebekozien O, Noor N, DiMeglio L, et al. 2022 state of type 1 diabetes in the U.S.—real world T1D exchange multicenter data from over 60,000 people. Diabetes. 2023;72(suppl 1):1456-P. doi:10.2337/db23-1456-P 19. American Diabetes Association Professional Practice Committee. 6. Glycemic Goals and Hypoglycemia: Standards of Care in Diabetes-2025. Diabetes Care. 2025;48(1)(suppl 1):S128-S145. doi:10.2337/dc25-S006 20. Gubitosi-Klug RA, Braffett BH, Hitt S, et al. Residual β cell function in long-term type 1 diabetes associates with reduced incidence of hypoglycemia. J Clin Invest. 2021;131(3):e143011. doi:10.1172/JCI143011 21. Östenson CG, Geelhoed-Duijvestijn P, Lahtela J, Weitgasser R, Jensen MM, Pedersen-Bjergaard U. Self-reported non-severe hypoglycaemic events in Europe. Diabet Med. 2014;31(1):92-101. doi:10.1111/dme.12261 22. Pedersen-Bjergaard U, Thorsteinsson B. Reporting severe hypoglycemia in type 1 diabetes: facts and pitfalls. Curr Diab Rep. 2017;17(12):131. doi:10.1007/s11892-017-0965-1 23. Jeyam A, Colhoun H, McGurnaghan S, et al. Clinical impact of residual C-peptide secretion in type 1 diabetes on glycemia and microvascular complications. Diabetes Care. 2021;44(2):390-398. doi:10.2337/dc20-0567 24. Hammersen J, Tittel SR, Warncke K, et al. Previous diabetic ketoacidosis as a risk factor for recurrence in a large prospective contemporary pediatric cohort: results from the DPV initiative. Pediatr Diabetes. 2021;22(3):455-462. doi:10.1111/pedi.13185 25. Lyerla R, Johnson-Rabbett B, Shakally A, Magar R, Alameddine H, Fish L. Recurrent DKA results in high societal costs – a retrospective study identifying social predictors of recurrence for potential future intervention. Clin Diabetes Endocrinol. 2021;7(1):13. doi:10.1186/s40842-021-00127-6 26. Harsunen M, Haukka J, Harjutsalo V, et al. Residual insulin secretion in individuals with type 1 diabetes in Finland: longitudinal and cross-sectional analyses. Lancet Diabetes Endocrinol. 2023;11(7):465-473. doi:10.1016/S2213-8587(23)00123-7 27. Ward K, Pan C, Shinde M, Rieuthavorn J, Hegde S, Gaebler JA. Modeling the Total Economic Value of Novel Type 1 Diabetes (T1D) Therapeutic Concepts. Health Advances. January 2020. Accessed June 6, 2025. https://t1dfund.org/wp-content/uploads/2020/02/Health-Advances-T1D-Concept-Value-White-Paper-2020.pdf 28. Leighton E, Sainsbury CA, Jones GC. A practical review of C-peptide testing in diabetes. Diabetes Ther. 2017;8(3):475-487. doi:10.1007/s13300-017-0265-4 29. Maddaloni E, Bolli GB, Frier BM, et al. C-peptide determination in the diagnosis of type of diabetes and its management: a clinical perspective. Diabetes Obes Metab. 2022;24(10):1912-1926. doi:10.1111/dom.14785 30. Stankute I, Dobrovolskiene R, Danyte E, Steponaviciute R, Schwitzgebel VM, Verkauskiene R. Pancreatic beta-cell function dynamics in youth with GCK, HNF1A, and KCNJ11 genes mutations during mixed meal tolerance test. Pediatr Diabetes. 2022;23(7):1009-1016. doi:10.1111/pedi.13404 31. Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med. 2013;30(7):803-817. doi:10.1111/dme.12159 32. Yang Y, Hua QX, Liu J, et al. Solution structure of proinsulin: connecting domain flexibility and prohormone processing. J Biol Chem. 2010;285(11):7847-7851. doi:10.1074/jbc.C109.084921 33. Palmer JP, Fleming GA, Greenbaum CJ, et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21-22 October 2001. Diabetes. 2004;53(1):250-264. doi:10.2337/diabetes.53.1.250 34. Design of Clinical Trials in New-Onset Type 1 Diabetes: Regulatory Considerations for Drug Development. Critical Path Institute (C-Path). June 15-16, 2021. Accessed June 6, 2025. https://media.c-path.org/wp-content/uploads/20240427170243/WorkshopSummary-1.pdf
This site is intended for US payers only.
© 2025 Sanofi. All rights reserved.