TZIELD: Clinical evidence
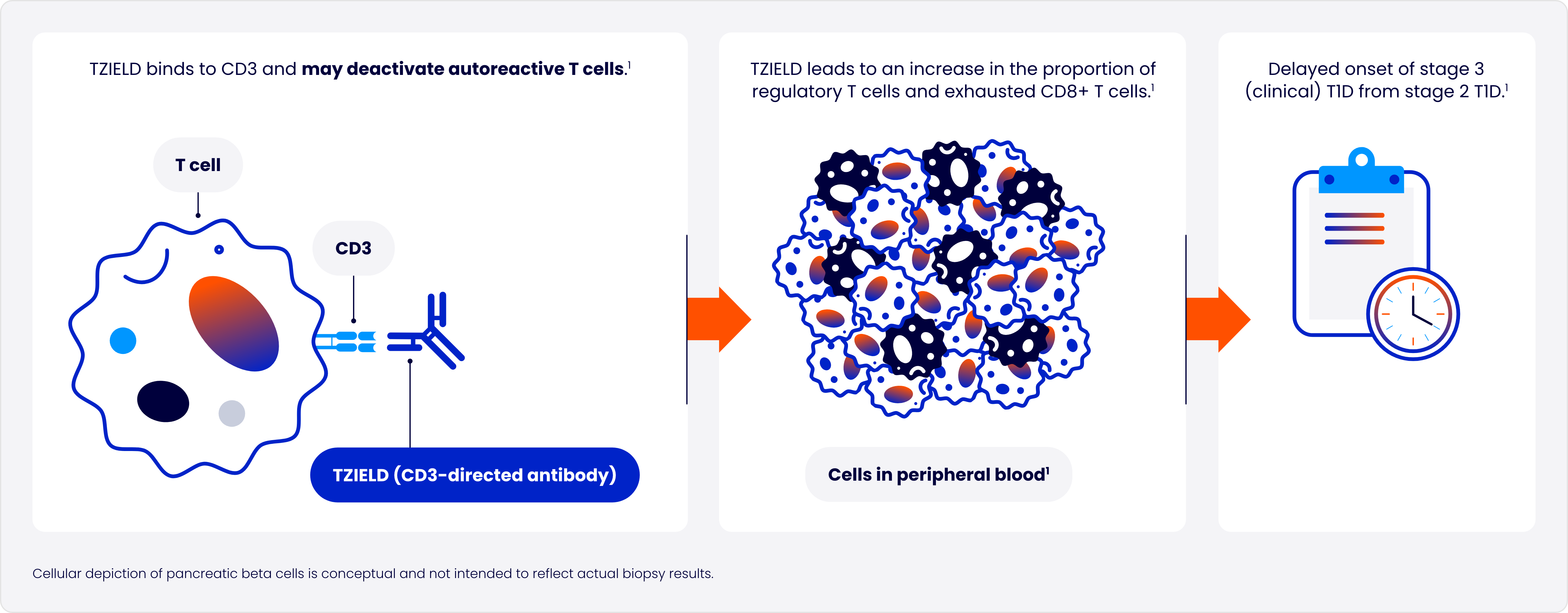
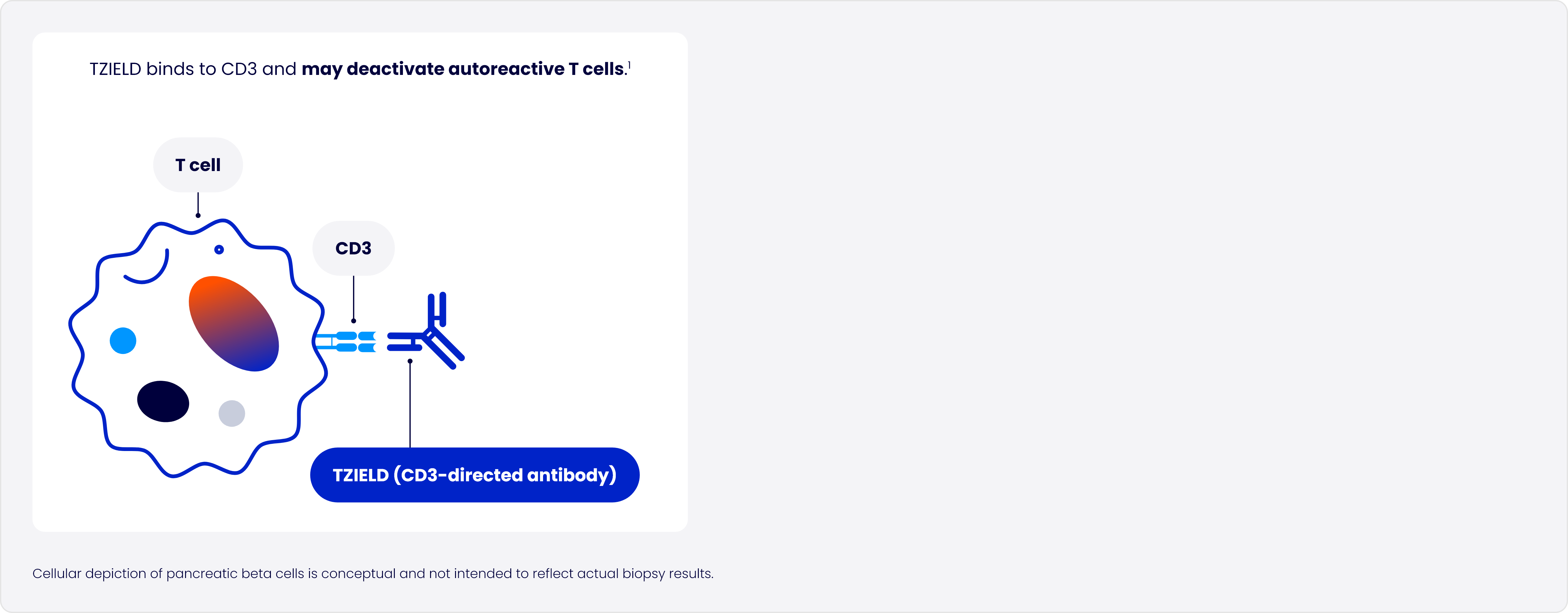
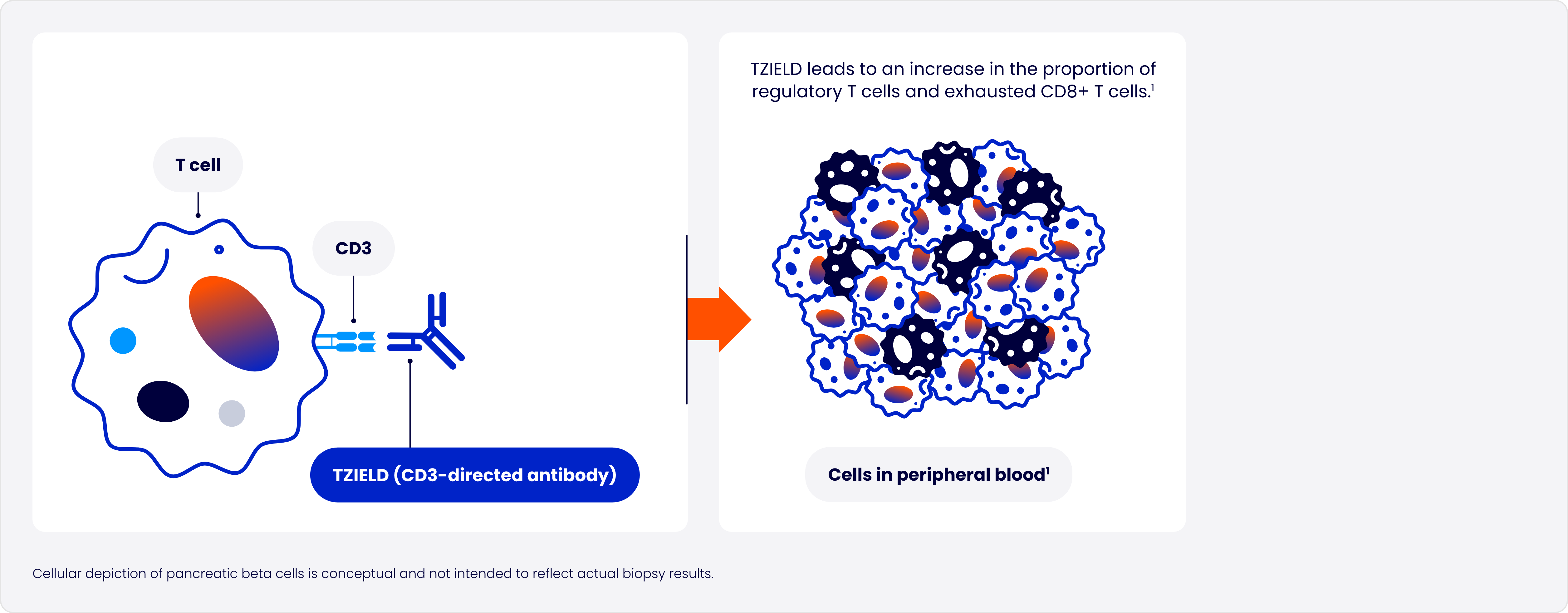
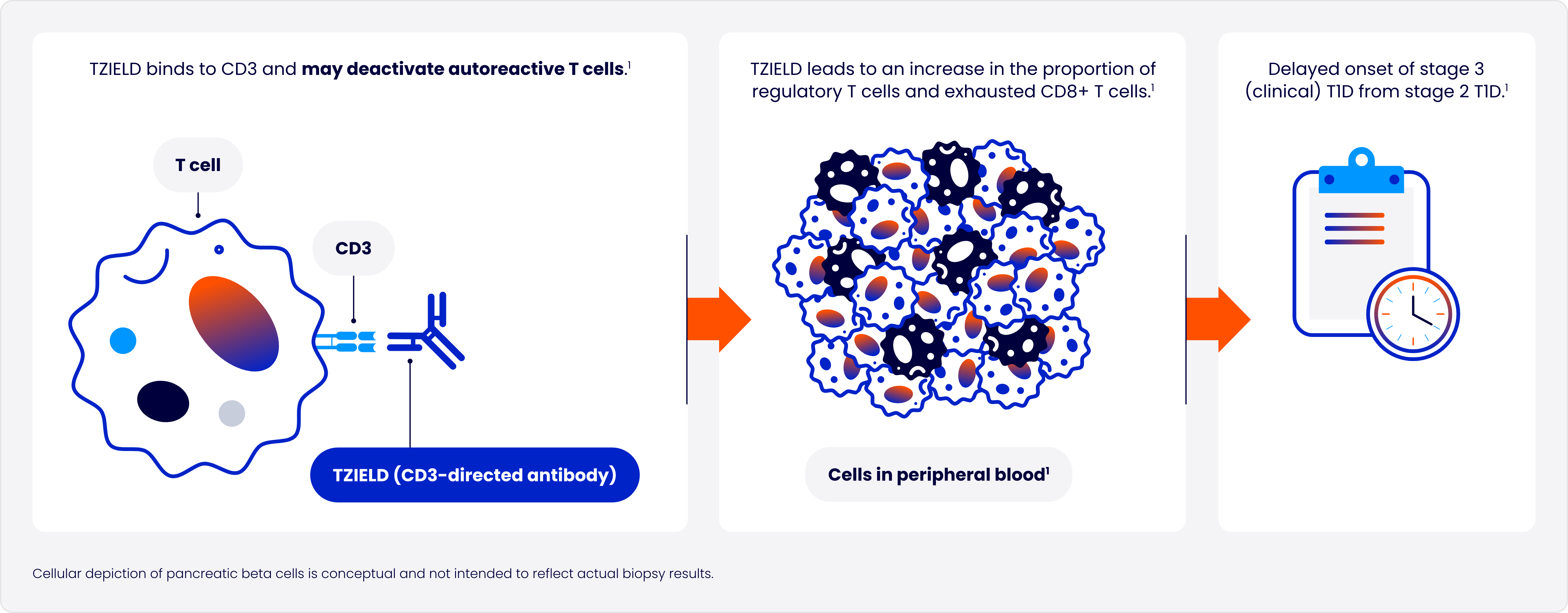
Designed to target the underlying autoimmune process of T1D
TZIELD is the first and only disease-modifying therapy approved to delay onset of stage 3 T1D in patients aged ≥8 years with stage 2 T1D1
In type 1 diabetes (T1D), autoreactive T cells of the immune system progressively destroy pancreatic beta cells2
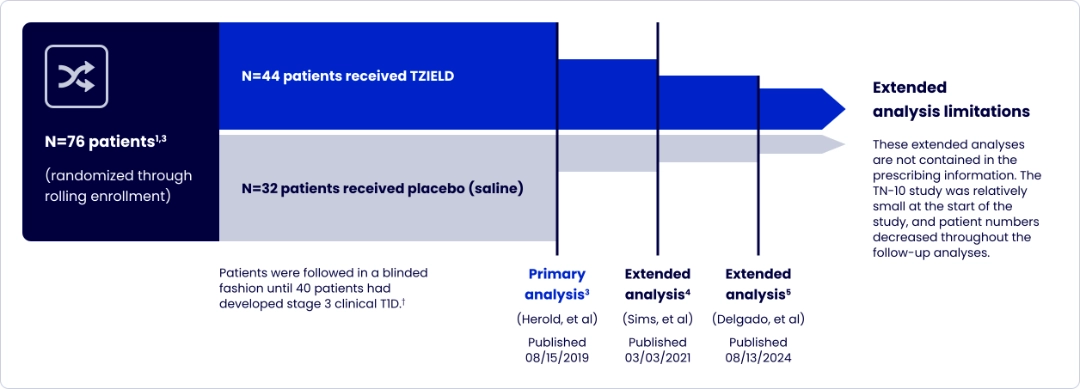
TZIELD was investigated in a phase 2, randomized, double-blind, event-driven, placebo-controlled study in 76 patients aged 8 to 49 years with stage 2 T1D1*
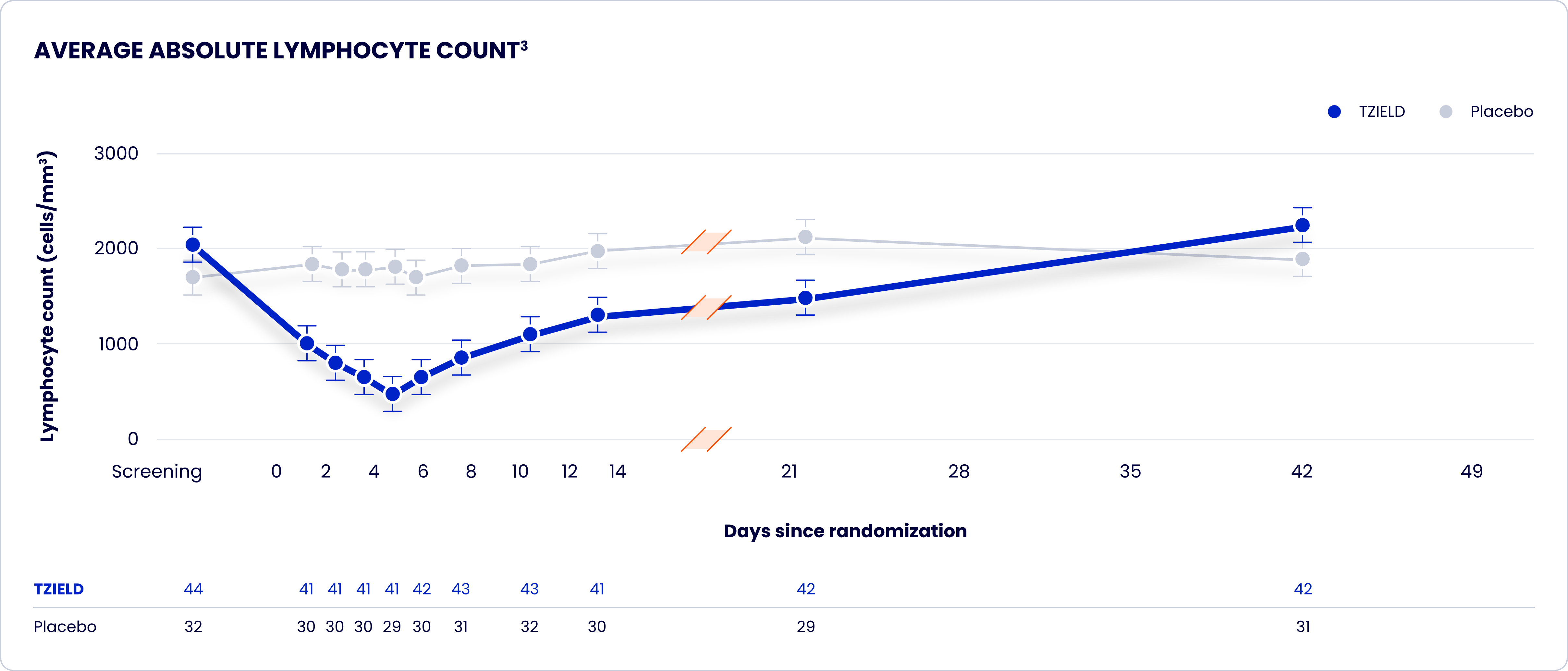
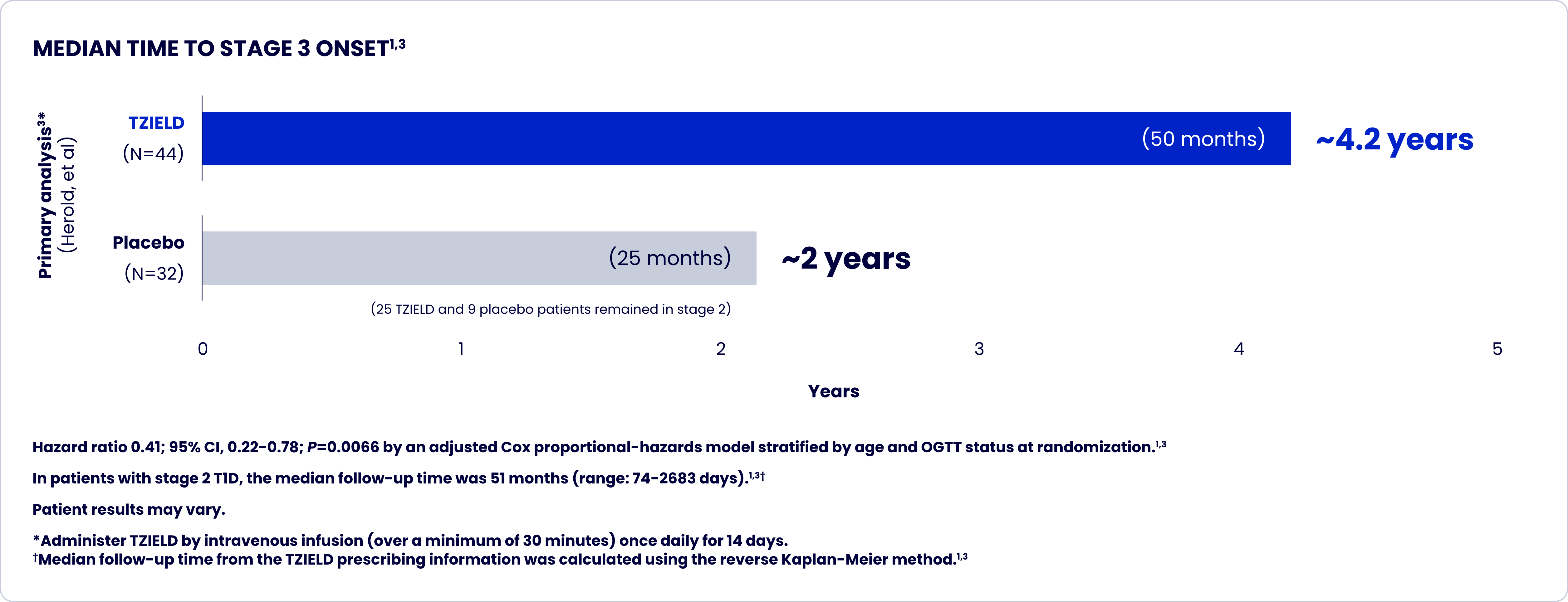
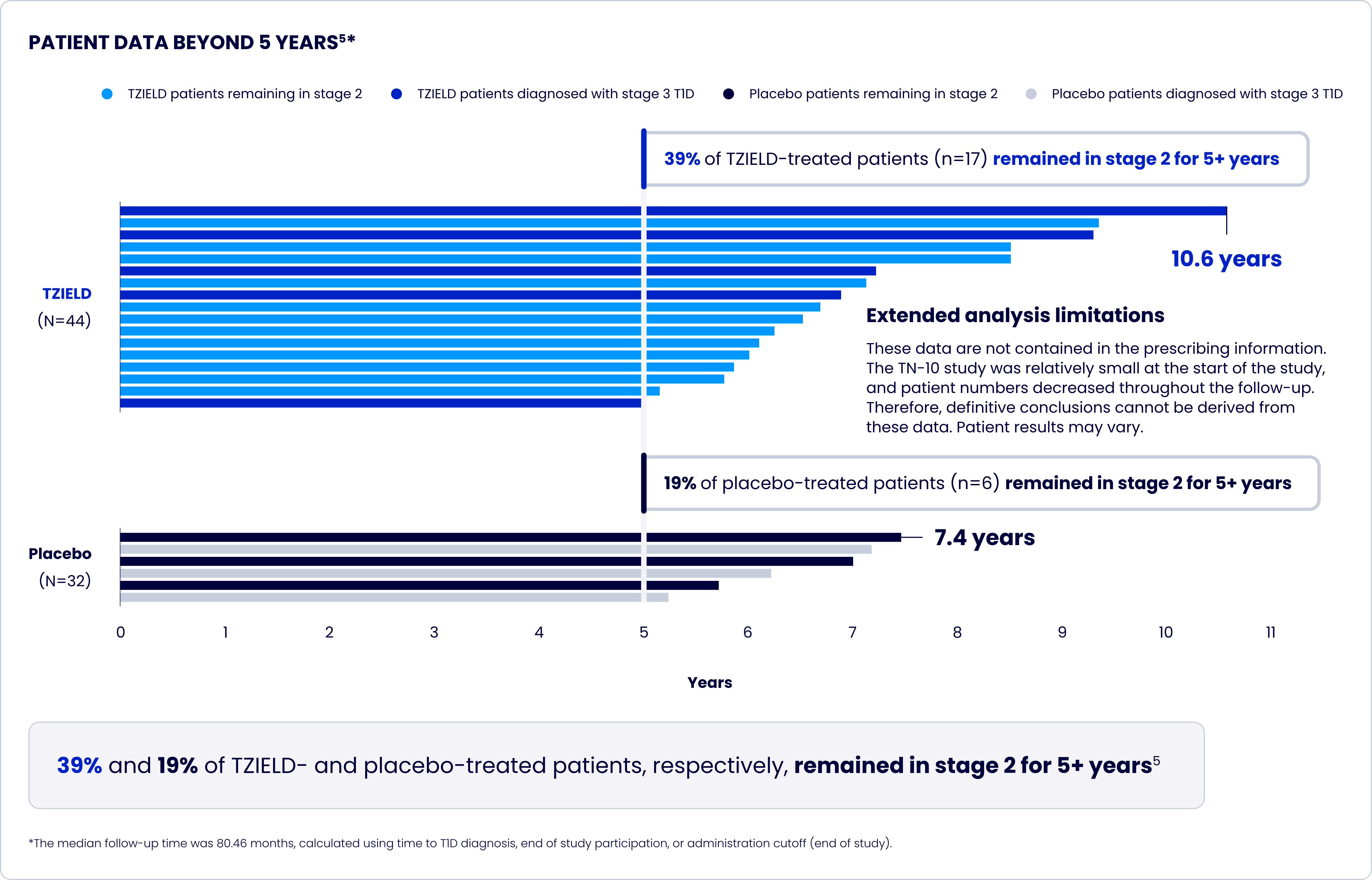
TrialNet-10 (TN-10) study design and extension analyses
One 2-week course of TZIELD doubled the median time to stage 3 onset vs placebo1*
TZIELD is an immunomodulator with a well-established safety profile1*
COMMON ADVERSE REACTIONS IN THE TN-10 STUDY†‡
| ADVERSE REACTION | TZIELD (N=44) | Placebo (N=32) |
| Lymphopenia | 73% | 6% |
| Rash§ | 36% | 0% |
| Leukopenia | 21% | 0% |
| Headache | 11% | 6% |
| Neutropenia | 5% | 3% |
| Alanine aminotransferase increaseda | 5% | 3% |
| Diarrheaa | 5% | 0% |
| Nasopharyngitis | 5% | 0% |
Please click below to see additional safety information for specific adverse events
*Adverse reactions in TZIELD-treated patients were also evaluated in a larger pool of adult and pediatric patients who participated in 5 controlled clinical studies (including the TN-10 study).
†Adverse reactions that occurred in 2 or more TZIELD-treated patients.
‡Adverse reactions that occurred during treatment and through 28 days after the last study drug administration.
§Composite of rash-related terms, including rash erythematous, rash macular, rash papular, rash maculo-papular, and rash pruritic.
∥In these studies, 436 patients received a 14-day dosing regimen of TZIELD with total drug exposure comparable to recommended approved dosage (Study TN-10). One hundred sixty-eight patients received a 14-day course of TZIELD with lower total TZIELD drug exposure, and 169 patients received a 6-day course of TZIELD with lower total TZIELD drug exposure.
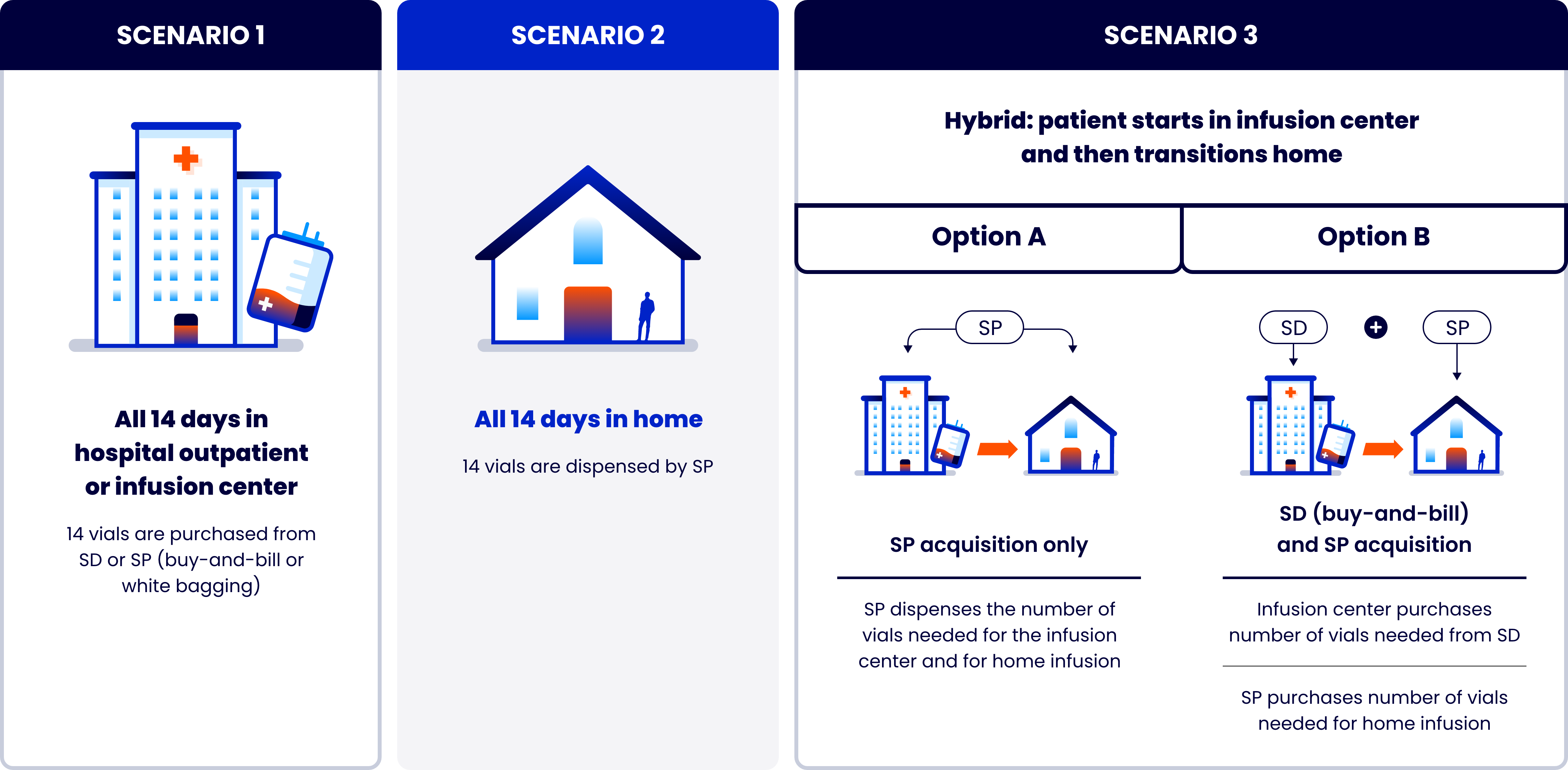
TZIELD is administered over the course of a single 2-week period1
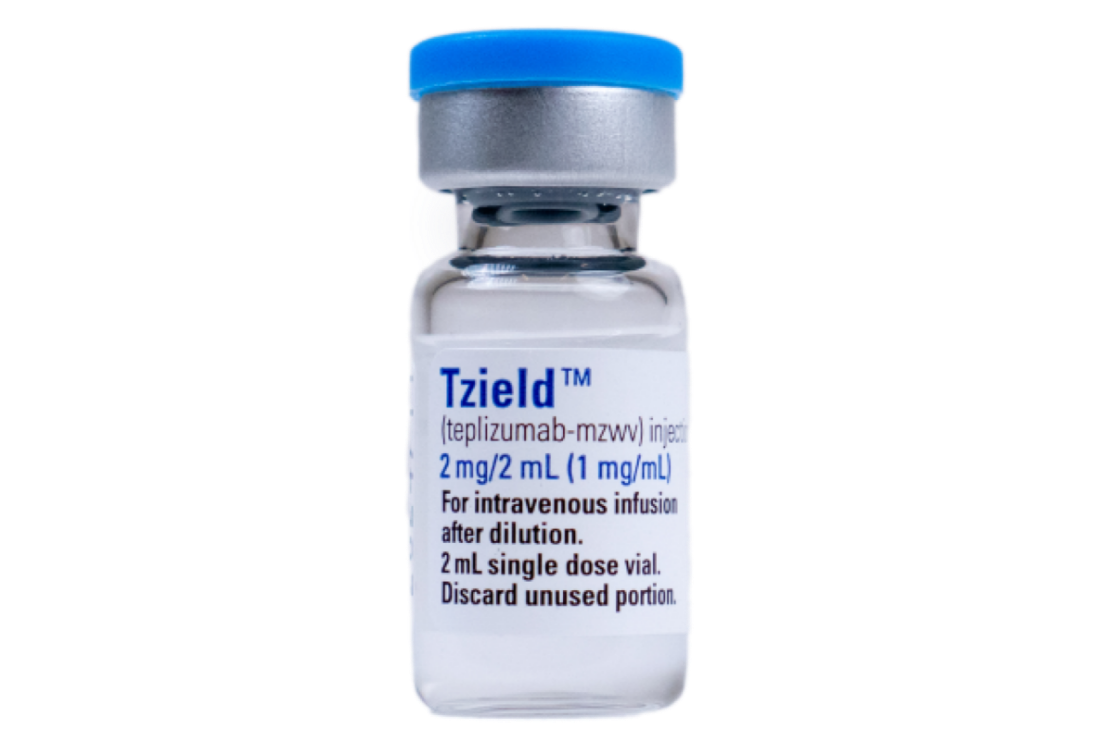
TZIELD injection: 2 mg/2 mL (1 mg/mL) clear and colorless solution in a single-dose vial1

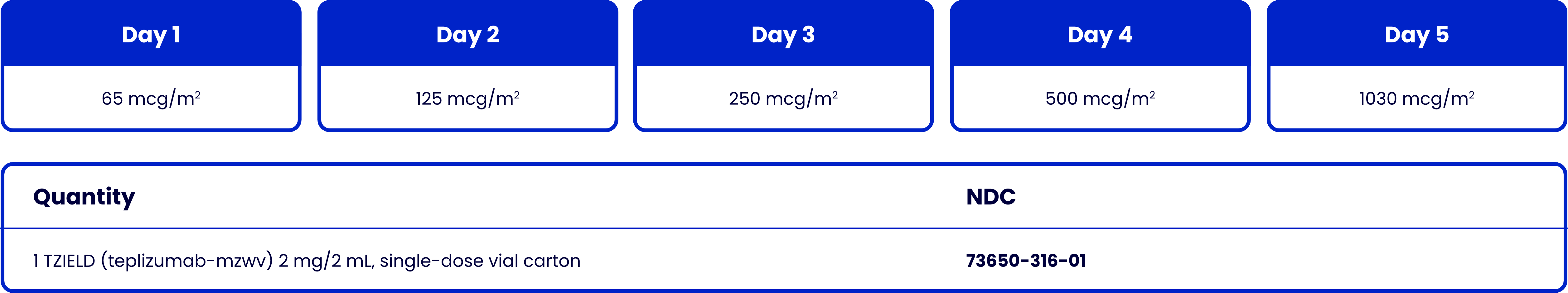
TZIELD is administered by intravenous infusion (over a minimum of 30 minutes), using body surface area (BSA)-based dosing1
TZIELD is given once daily for 14 consecutive days1*
Recommended dosing schedule1
Based on BSA dosing requirements, 2 vials may be needed for some individuals (BSA >1.94 m2) for Days 5 to 14.10
*Do not administer 2 doses on the same day. If a planned TZIELD infusion is missed, resume dosing by administering all remaining doses on consecutive days to complete the 14-day treatment course.
NDC, National Drug Code.
References: 1. TZIELD. Prescribing information. Provention Bio, Inc.; 2023. 2. DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391(10138):2449-2462. doi:10.1016/S0140-6736(18)31320-5 3. Herold KC, Bundy BN, Long SA, et al. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med. 2019;381(7):603-613. doi:10.1056/NEJMoa1902226 4. Sims EK, Bundy BN, Stier K, et al. Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals. Sci Transl Med. 2021;13(583):eabc8980. doi:10.1126/scitranslmed.abc8980 5. Lledó-Delgado A, Preston-Hurlburt P, Currie S, et al. Teplizumab induces persistent changes in the antigen-specific repertoire in individuals at risk for type 1 diabetes. J Clin Invest. 2024;134(18):e177492. doi:10.1172/JCI177492 6. Teplizumab BLA 761183 clinical review. Center for Drug Evaluation and Research; US Food and Drug Administration. July 2, 2021. 7. Data on file. Sanofi Biotechnology. 2024. 8. Baumgart DC, Lowder JN, Targan SR, Sandborn WJ, Frankel MB. Transient cytokine-induced liver injury following administration of the humanized anti-CD3 antibody visilizumab (HuM291) in Crohn’s disease. Am J Gastroenterol. 2009;104(4):868-876. doi:10.1038/ajg.2008.138 9. Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. doi:10.1186/s40425-018-0343-9 10. Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098. doi:10.1056/NEJM198710223171717 11. American Diabetes Association Professional Practice Committee. Prevention or delay of diabetes and associated comorbidities: standards of care in diabetes-2025. Diabetes Care. 2025;48(suppl 1):S50-S58. doi:10.2337/dc25-S003
INDICATION AND IMPORTANT SAFTEY INFORMATION
TZIELD® (teplizumab-mzwv) is a CD3-directed monoclonal antibody indicated to delay the onset of Stage 3 type 1 diabetes (T1D) in adults and pediatric patients aged 8 years and older with Stage 2 T1D.
WARNINGS AND PRECAUTIONS
- Cytokine Release Syndrome (CRS): CRS occurred in TZIELD-treated patients during the treatment period and through 28 days after the last drug administration. Prior to TZIELD treatment, premedicate with antipyretics, antihistamines and/or antiemetics, and treat similarly if symptoms occur during treatment. If severe CRS develops, consider pausing dosing for 1 day to 2 days and administering the remaining doses to complete the full 14-day course on consecutive days; or discontinue treatment. Monitor liver enzymes during treatment. Discontinue TZIELD treatment in patients who develop elevated alanine aminotransferase or aspartate aminotransferase more than 5 times the upper limit of normal (ULN) or bilirubin more than 3 times ULN.
- Serious Infections: Use of TZIELD is not recommended in patients with active serious infection or chronic infection other than localized skin infections. Monitor patients for signs and symptoms of infection during and after TZIELD administration. If serious infection develops, treat appropriately, and discontinue TZIELD.
- Lymphopenia: Lymphopenia occurred in most TZIELD-treated patients. For most patients, lymphocyte levels began to recover after the fifth day of treatment and returned to pretreatment values within two weeks after treatment completion and without dose interruption. Monitor white blood cell counts during the treatment period. If prolonged severe lymphopenia develops (<500 cells per mcL lasting 1 week or longer), discontinue TZIELD.
- Hypersensitivity Reactions: Acute hypersensitivity reactions including serum sickness, angioedema, urticaria, rash, vomiting and bronchospasm occurred in TZIELD-treated patients. If severe hypersensitivity reactions occur, discontinue TZIELD and treat promptly.
- Vaccinations: The safety of immunization with live-attenuated (live) vaccines with TZIELD-treated patients has not been studied. TZIELD may interfere with immune response to vaccination and decrease vaccine efficacy. Administer all age-appropriate vaccinations prior to starting TZIELD.
- Administer live vaccines at least 8 weeks prior to treatment. Live vaccines are not recommended during treatment, or up to 52 weeks after treatment.
- Administer inactivated (killed) vaccines or mRNA vaccines at least 2 weeks prior to treatment. Inactivated vaccines are not recommended during treatment or 6 weeks after completion of treatment.
Adverse Reactions:
Most common adverse reactions (>10%) were lymphopenia, rash, leukopenia, and headache.
USE IN SPECIFIC POPULATIONS
- Pregnancy: May cause fetal harm.
- Lactation: A lactating woman may consider pumping and discarding breast milk during and for 20 days after TZIELD administration.
Please see full Prescribing Information, including patient selection criteria, and Medication Guide. View Important Safety Information page.
INDICATION AND IMPORTANT SAFTEY INFORMATION
TZIELD® (teplizumab-mzwv) is a CD3-directed monoclonal antibody indicated to delay the onset of Stage 3 type 1 diabetes (T1D) in adults and pediatric patients aged 8 years and older with Stage 2 T1D.
WARNINGS AND PRECAUTIONS
- Cytokine Release Syndrome (CRS): CRS occurred in TZIELD-treated patients during the treatment period and through 28 days after the last drug administration. Prior to TZIELD treatment, premedicate with antipyretics, antihistamines and/or antiemetics, and treat similarly if symptoms occur during treatment. If severe CRS develops, consider pausing dosing for 1 day to 2 days and administering the remaining doses to complete the full 14-day course on consecutive days; or discontinue treatment. Monitor liver enzymes during treatment. Discontinue TZIELD treatment in patients who develop elevated alanine aminotransferase or aspartate aminotransferase more than 5 times the upper limit of normal (ULN) or bilirubin more than 3 times ULN.
- Serious Infections: Use of TZIELD is not recommended in patients with active serious infection or chronic infection other than localized skin infections. Monitor patients for signs and symptoms of infection during and after TZIELD administration. If serious infection develops, treat appropriately, and discontinue TZIELD.
- Lymphopenia: Lymphopenia occurred in most TZIELD-treated patients. For most patients, lymphocyte levels began to recover after the fifth day of treatment and returned to pretreatment values within two weeks after treatment completion and without dose interruption. Monitor white blood cell counts during the treatment period. If prolonged severe lymphopenia develops (<500 cells per mcL lasting 1 week or longer), discontinue TZIELD.
- Hypersensitivity Reactions: Acute hypersensitivity reactions including serum sickness, angioedema, urticaria, rash, vomiting and bronchospasm occurred in TZIELD-treated patients. If severe hypersensitivity reactions occur, discontinue TZIELD and treat promptly.
- Vaccinations: The safety of immunization with live-attenuated (live) vaccines with TZIELD-treated patients has not been studied. TZIELD may interfere with immune response to vaccination and decrease vaccine efficacy. Administer all age-appropriate vaccinations prior to starting TZIELD.
- Administer live vaccines at least 8 weeks prior to treatment. Live vaccines are not recommended during treatment, or up to 52 weeks after treatment.
- Administer inactivated (killed) vaccines or mRNA vaccines at least 2 weeks prior to treatment. Inactivated vaccines are not recommended during treatment or 6 weeks after completion of treatment.
Adverse Reactions:
Most common adverse reactions (>10%) were lymphopenia, rash, leukopenia, and headache.
USE IN SPECIFIC POPULATIONS
- Pregnancy: May cause fetal harm.
- Lactation: A lactating woman may consider pumping and discarding breast milk during and for 20 days after TZIELD administration.
Please see full Prescribing Information, including patient selection criteria, and Medication Guide. View Important Safety Information page.
INDICATION AND IMPORTANT SAFTEY INFORMATION
TZIELD® (teplizumab-mzwv) is a CD3-directed monoclonal antibody indicated to delay the onset of Stage 3 type 1 diabetes (T1D) in adults and pediatric patients aged 8 years and older with Stage 2 T1D.
WARNINGS AND PRECAUTIONS
- Cytokine Release Syndrome (CRS): CRS occurred in TZIELD-treated patients during the treatment period and through 28 days after the last drug administration. Prior to TZIELD treatment, premedicate with antipyretics, antihistamines and/or antiemetics, and treat similarly if symptoms occur during treatment. If severe CRS develops, consider pausing dosing for 1 day to 2 days and administering the remaining doses to complete the full 14-day course on consecutive days; or discontinue treatment. Monitor liver enzymes during treatment. Discontinue TZIELD treatment in patients who develop elevated alanine aminotransferase or aspartate aminotransferase more than 5 times the upper limit of normal (ULN) or bilirubin more than 3 times ULN.
- Serious Infections: Use of TZIELD is not recommended in patients with active serious infection or chronic infection other than localized skin infections. Monitor patients for signs and symptoms of infection during and after TZIELD administration. If serious infection develops, treat appropriately, and discontinue TZIELD.
- Lymphopenia: Lymphopenia occurred in most TZIELD-treated patients. For most patients, lymphocyte levels began to recover after the fifth day of treatment and returned to pretreatment values within two weeks after treatment completion and without dose interruption. Monitor white blood cell counts during the treatment period. If prolonged severe lymphopenia develops (<500 cells per mcL lasting 1 week or longer), discontinue TZIELD.
- Hypersensitivity Reactions: Acute hypersensitivity reactions including serum sickness, angioedema, urticaria, rash, vomiting and bronchospasm occurred in TZIELD-treated patients. If severe hypersensitivity reactions occur, discontinue TZIELD and treat promptly.
- Vaccinations: The safety of immunization with live-attenuated (live) vaccines with TZIELD-treated patients has not been studied. TZIELD may interfere with immune response to vaccination and decrease vaccine efficacy. Administer all age-appropriate vaccinations prior to starting TZIELD.
- Administer live vaccines at least 8 weeks prior to treatment. Live vaccines are not recommended during treatment, or up to 52 weeks after treatment.
- Administer inactivated (killed) vaccines or mRNA vaccines at least 2 weeks prior to treatment. Inactivated vaccines are not recommended during treatment or 6 weeks after completion of treatment.
Adverse Reactions:
Most common adverse reactions (>10%) were lymphopenia, rash, leukopenia, and headache.
USE IN SPECIFIC POPULATIONS
- Pregnancy: May cause fetal harm.
- Lactation: A lactating woman may consider pumping and discarding breast milk during and for 20 days after TZIELD administration.
Please see full Prescribing Information, including patient selection criteria, and Medication Guide. View Important Safety Information page.
This site is intended for US payers only.
© Sanofi. All rights reserved.
TZIELD is a registered trademarks of Sanofi or an affiliate.



![[callout 1] Text callout highlighting CRS can be mitigated by premedicating with antipyretics, antihistamines, and/or antiemetics, or by pausing dosing [callout 2] Text callout highlighting premedicate, monitor liver enzymes, discontinue in those who develop elevated ALT or AST levels >5x the ULN, and, if severe CRS develops, consider temporarily pausing dosing](/.imaging/webp/sanofi-templates/w1080/dam/market-access-com/products/specialty-care/Tzield/TZIELD-double-callouts-pop-up-image-DT.png/jcr:content/TZIELD-double-callouts-pop-up-image-DT.png)