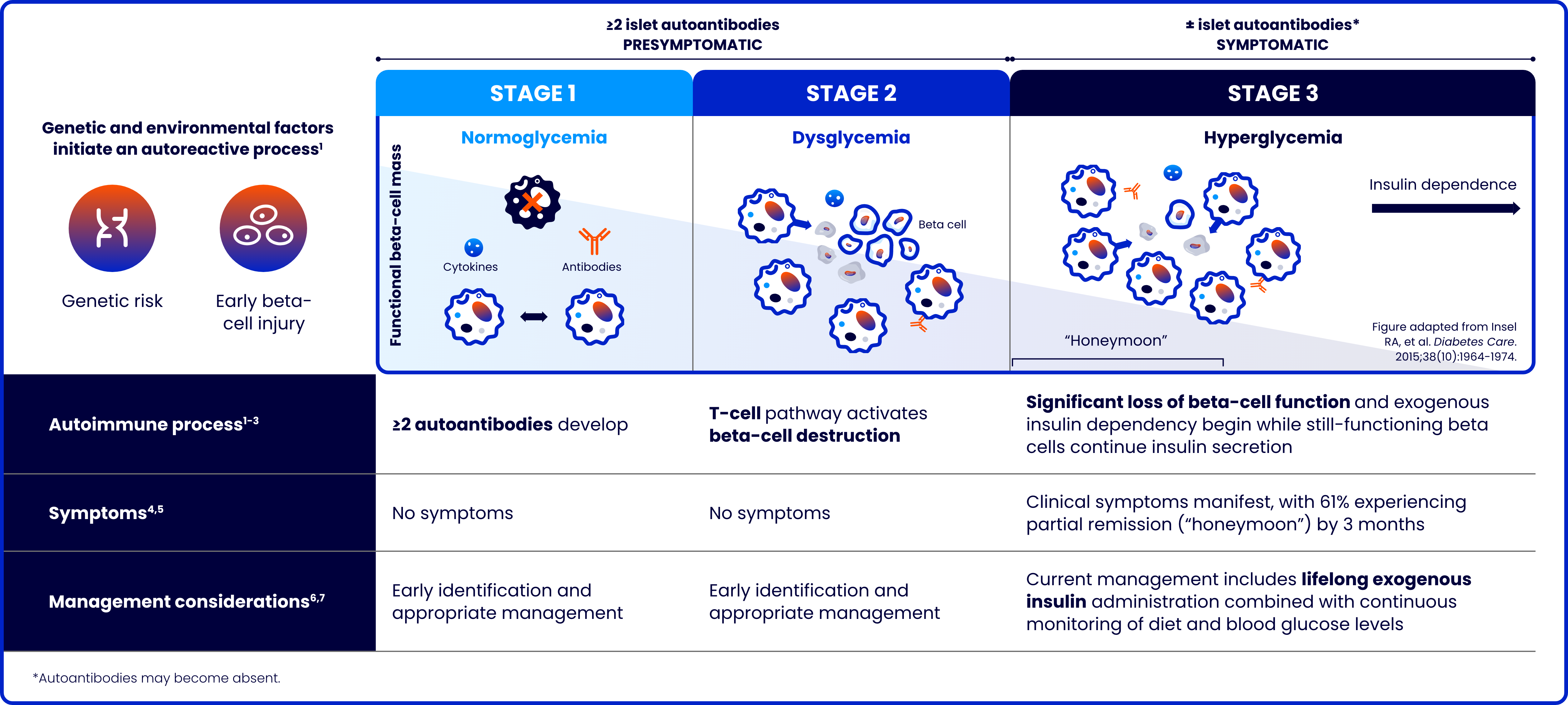
Type 1 diabetes: A progressive autoimmune disease with lifelong consequences
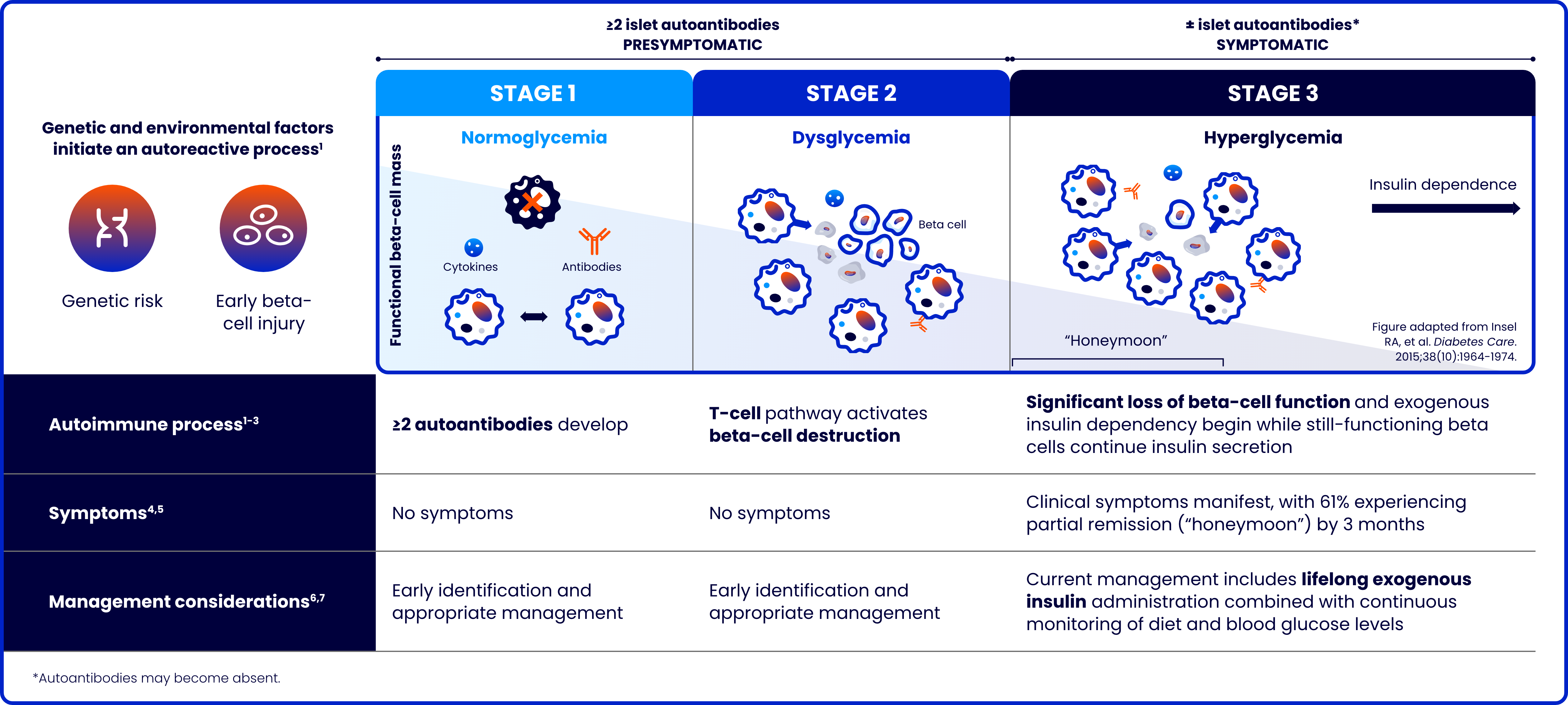
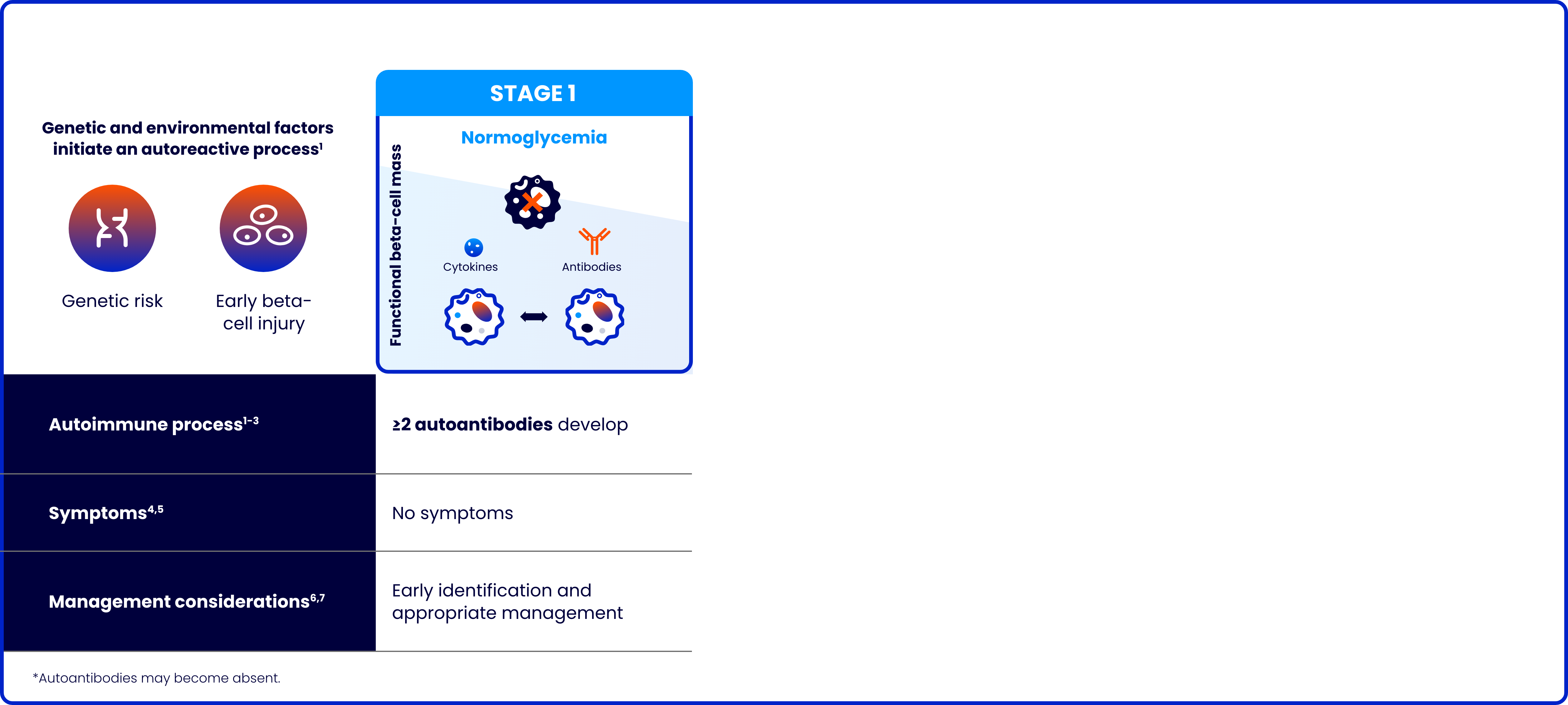
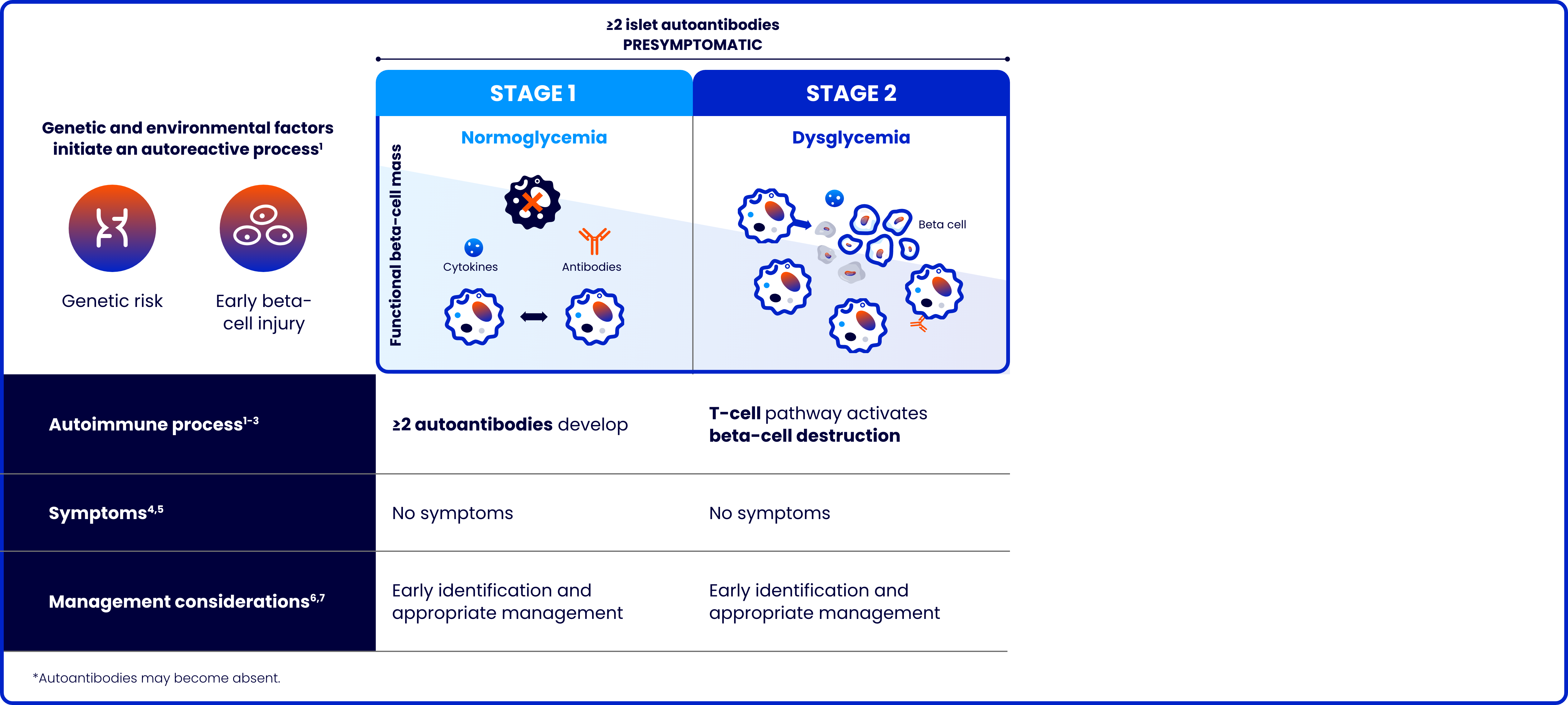
Type 1 diabetes (T1D) is a progressive autoimmune disease characterized by the destruction of pancreatic beta cells
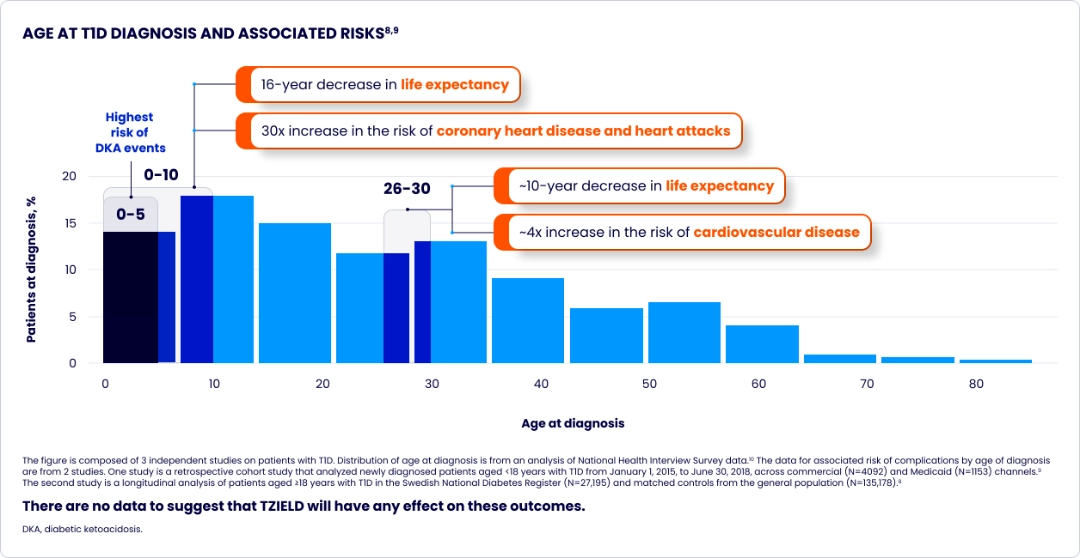
The risk of complications increases the younger the age at stage 3 diagnosis8-10
ADA, American Diabetes Association.
References: 1. Skyler JS, Bakris GL, Bonifacio E, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. 2017;66(2):241-255. doi:10.2337/db16-0806 2. Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38(10):1964-1974. doi:10.2337/dc15-1419 3. DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391(10138):2449-2462. doi:10.1016/S0140-6736(18)31320-5 4. American Diabetes Association Professional Practice Committee. Prevention or delay of diabetes and associated comorbidities: standards of care in diabetes-2025. Diabetes Care. 2025;48(suppl 1):S50-S58. doi:10.2337/dc25-S003 5. Mortensen HB, Hougaard P, Swift P, et al. New definition for the partial remission period in children and adolescents with type 1 diabetes. Diabetes Care. 2009;32(8):1384-1390. doi:10.2337/dc08-1987 6. Haller MJ, Bell KJ, Besser REJ, et al. ISPAD Clinical Practice Consensus Guidelines 2024: Screening, staging, and strategies to preserve beta-cell function in children and adolescents with type 1 diabetes. Horm Res Paediatr. 2024;97(6):529-545. doi:10.1159/000543035 7. Sundheim B, Hirani K, Blaschke M, Lemos JRN, Mittal R. Pre-type 1 diabetes in adolescents and teens: screening, nutritional interventions, beta-cell preservation, and psychosocial impacts. J Clin Med. 2025;14(2):383. doi:10.3390/jcm14020383 8. Rawshani A, Sattar N, Franzén S, et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet. 2018;392(10146):477-486. doi:10.1016/S0140-6736(18)31506-X 9. Cagle A, Stokes M, Li Q, et al. Increased burden of diabetic ketoacidosis among pediatric patients with type 1 diabetes using Medicaid in the United States. Poster presented at: AMCP 2024 Annual Meeting; April 15-18, 2024; New Orleans, LA. 10. Fang M, Wang D, Echouffo-Tcheugui JB, Selvin E. Age at diagnosis in U.S. adults with type 1 diabetes. Ann Intern Med. 2023;176(11):1567-1568. doi:10.7326/M23-1707
INDICATION AND IMPORTANT SAFTEY INFORMATION
TZIELD® (teplizumab-mzwv) is a CD3-directed monoclonal antibody indicated to delay the onset of Stage 3 type 1 diabetes (T1D) in adults and pediatric patients aged 8 years and older with Stage 2 T1D.
WARNINGS AND PRECAUTIONS
- Cytokine Release Syndrome (CRS): CRS occurred in TZIELD-treated patients during the treatment period and through 28 days after the last drug administration. Prior to TZIELD treatment, premedicate with antipyretics, antihistamines and/or antiemetics, and treat similarly if symptoms occur during treatment. If severe CRS develops, consider pausing dosing for 1 day to 2 days and administering the remaining doses to complete the full 14-day course on consecutive days; or discontinue treatment. Monitor liver enzymes during treatment. Discontinue TZIELD treatment in patients who develop elevated alanine aminotransferase or aspartate aminotransferase more than 5 times the upper limit of normal (ULN) or bilirubin more than 3 times ULN.
- Serious Infections: Use of TZIELD is not recommended in patients with active serious infection or chronic infection other than localized skin infections. Monitor patients for signs and symptoms of infection during and after TZIELD administration. If serious infection develops, treat appropriately, and discontinue TZIELD.
- Lymphopenia: Lymphopenia occurred in most TZIELD-treated patients. For most patients, lymphocyte levels began to recover after the fifth day of treatment and returned to pretreatment values within two weeks after treatment completion and without dose interruption. Monitor white blood cell counts during the treatment period. If prolonged severe lymphopenia develops (<500 cells per mcL lasting 1 week or longer), discontinue TZIELD.
- Hypersensitivity Reactions: Acute hypersensitivity reactions including serum sickness, angioedema, urticaria, rash, vomiting and bronchospasm occurred in TZIELD-treated patients. If severe hypersensitivity reactions occur, discontinue TZIELD and treat promptly.
- Vaccinations: The safety of immunization with live-attenuated (live) vaccines with TZIELD-treated patients has not been studied. TZIELD may interfere with immune response to vaccination and decrease vaccine efficacy. Administer all age-appropriate vaccinations prior to starting TZIELD.
- Administer live vaccines at least 8 weeks prior to treatment. Live vaccines are not recommended during treatment, or up to 52 weeks after treatment.
- Administer inactivated (killed) vaccines or mRNA vaccines at least 2 weeks prior to treatment. Inactivated vaccines are not recommended during treatment or 6 weeks after completion of treatment.
Adverse Reactions:
Most common adverse reactions (>10%) were lymphopenia, rash, leukopenia, and headache.
USE IN SPECIFIC POPULATIONS
- Pregnancy: May cause fetal harm.
- Lactation: A lactating woman may consider pumping and discarding breast milk during and for 20 days after TZIELD administration.
Please see full Prescribing Information, including patient selection criteria, and Medication Guide. View Important Safety Information page.
INDICATION AND IMPORTANT SAFTEY INFORMATION
TZIELD® (teplizumab-mzwv) is a CD3-directed monoclonal antibody indicated to delay the onset of Stage 3 type 1 diabetes (T1D) in adults and pediatric patients aged 8 years and older with Stage 2 T1D.
WARNINGS AND PRECAUTIONS
- Cytokine Release Syndrome (CRS): CRS occurred in TZIELD-treated patients during the treatment period and through 28 days after the last drug administration. Prior to TZIELD treatment, premedicate with antipyretics, antihistamines and/or antiemetics, and treat similarly if symptoms occur during treatment. If severe CRS develops, consider pausing dosing for 1 day to 2 days and administering the remaining doses to complete the full 14-day course on consecutive days; or discontinue treatment. Monitor liver enzymes during treatment. Discontinue TZIELD treatment in patients who develop elevated alanine aminotransferase or aspartate aminotransferase more than 5 times the upper limit of normal (ULN) or bilirubin more than 3 times ULN.
- Serious Infections: Use of TZIELD is not recommended in patients with active serious infection or chronic infection other than localized skin infections. Monitor patients for signs and symptoms of infection during and after TZIELD administration. If serious infection develops, treat appropriately, and discontinue TZIELD.
- Lymphopenia: Lymphopenia occurred in most TZIELD-treated patients. For most patients, lymphocyte levels began to recover after the fifth day of treatment and returned to pretreatment values within two weeks after treatment completion and without dose interruption. Monitor white blood cell counts during the treatment period. If prolonged severe lymphopenia develops (<500 cells per mcL lasting 1 week or longer), discontinue TZIELD.
- Hypersensitivity Reactions: Acute hypersensitivity reactions including serum sickness, angioedema, urticaria, rash, vomiting and bronchospasm occurred in TZIELD-treated patients. If severe hypersensitivity reactions occur, discontinue TZIELD and treat promptly.
- Vaccinations: The safety of immunization with live-attenuated (live) vaccines with TZIELD-treated patients has not been studied. TZIELD may interfere with immune response to vaccination and decrease vaccine efficacy. Administer all age-appropriate vaccinations prior to starting TZIELD.
- Administer live vaccines at least 8 weeks prior to treatment. Live vaccines are not recommended during treatment, or up to 52 weeks after treatment.
- Administer inactivated (killed) vaccines or mRNA vaccines at least 2 weeks prior to treatment. Inactivated vaccines are not recommended during treatment or 6 weeks after completion of treatment.
Adverse Reactions:
Most common adverse reactions (>10%) were lymphopenia, rash, leukopenia, and headache.
USE IN SPECIFIC POPULATIONS
- Pregnancy: May cause fetal harm.
- Lactation: A lactating woman may consider pumping and discarding breast milk during and for 20 days after TZIELD administration.
Please see full Prescribing Information, including patient selection criteria, and Medication Guide. View Important Safety Information page.
INDICATION AND IMPORTANT SAFTEY INFORMATION
TZIELD® (teplizumab-mzwv) is a CD3-directed monoclonal antibody indicated to delay the onset of Stage 3 type 1 diabetes (T1D) in adults and pediatric patients aged 8 years and older with Stage 2 T1D.
WARNINGS AND PRECAUTIONS
- Cytokine Release Syndrome (CRS): CRS occurred in TZIELD-treated patients during the treatment period and through 28 days after the last drug administration. Prior to TZIELD treatment, premedicate with antipyretics, antihistamines and/or antiemetics, and treat similarly if symptoms occur during treatment. If severe CRS develops, consider pausing dosing for 1 day to 2 days and administering the remaining doses to complete the full 14-day course on consecutive days; or discontinue treatment. Monitor liver enzymes during treatment. Discontinue TZIELD treatment in patients who develop elevated alanine aminotransferase or aspartate aminotransferase more than 5 times the upper limit of normal (ULN) or bilirubin more than 3 times ULN.
- Serious Infections: Use of TZIELD is not recommended in patients with active serious infection or chronic infection other than localized skin infections. Monitor patients for signs and symptoms of infection during and after TZIELD administration. If serious infection develops, treat appropriately, and discontinue TZIELD.
- Lymphopenia: Lymphopenia occurred in most TZIELD-treated patients. For most patients, lymphocyte levels began to recover after the fifth day of treatment and returned to pretreatment values within two weeks after treatment completion and without dose interruption. Monitor white blood cell counts during the treatment period. If prolonged severe lymphopenia develops (<500 cells per mcL lasting 1 week or longer), discontinue TZIELD.
- Hypersensitivity Reactions: Acute hypersensitivity reactions including serum sickness, angioedema, urticaria, rash, vomiting and bronchospasm occurred in TZIELD-treated patients. If severe hypersensitivity reactions occur, discontinue TZIELD and treat promptly.
- Vaccinations: The safety of immunization with live-attenuated (live) vaccines with TZIELD-treated patients has not been studied. TZIELD may interfere with immune response to vaccination and decrease vaccine efficacy. Administer all age-appropriate vaccinations prior to starting TZIELD.
- Administer live vaccines at least 8 weeks prior to treatment. Live vaccines are not recommended during treatment, or up to 52 weeks after treatment.
- Administer inactivated (killed) vaccines or mRNA vaccines at least 2 weeks prior to treatment. Inactivated vaccines are not recommended during treatment or 6 weeks after completion of treatment.
Adverse Reactions:
Most common adverse reactions (>10%) were lymphopenia, rash, leukopenia, and headache.
USE IN SPECIFIC POPULATIONS
- Pregnancy: May cause fetal harm.
- Lactation: A lactating woman may consider pumping and discarding breast milk during and for 20 days after TZIELD administration.
Please see full Prescribing Information, including patient selection criteria, and Medication Guide. View Important Safety Information page.
This site is intended for US payers only.
© Sanofi. All rights reserved.
TZIELD is a registered trademarks of Sanofi or an affiliate.