QFITLIA: CLINICAL EVIDENCE
How a treatment impacts the underlying condition is important when evaluating the clinical value that it brings
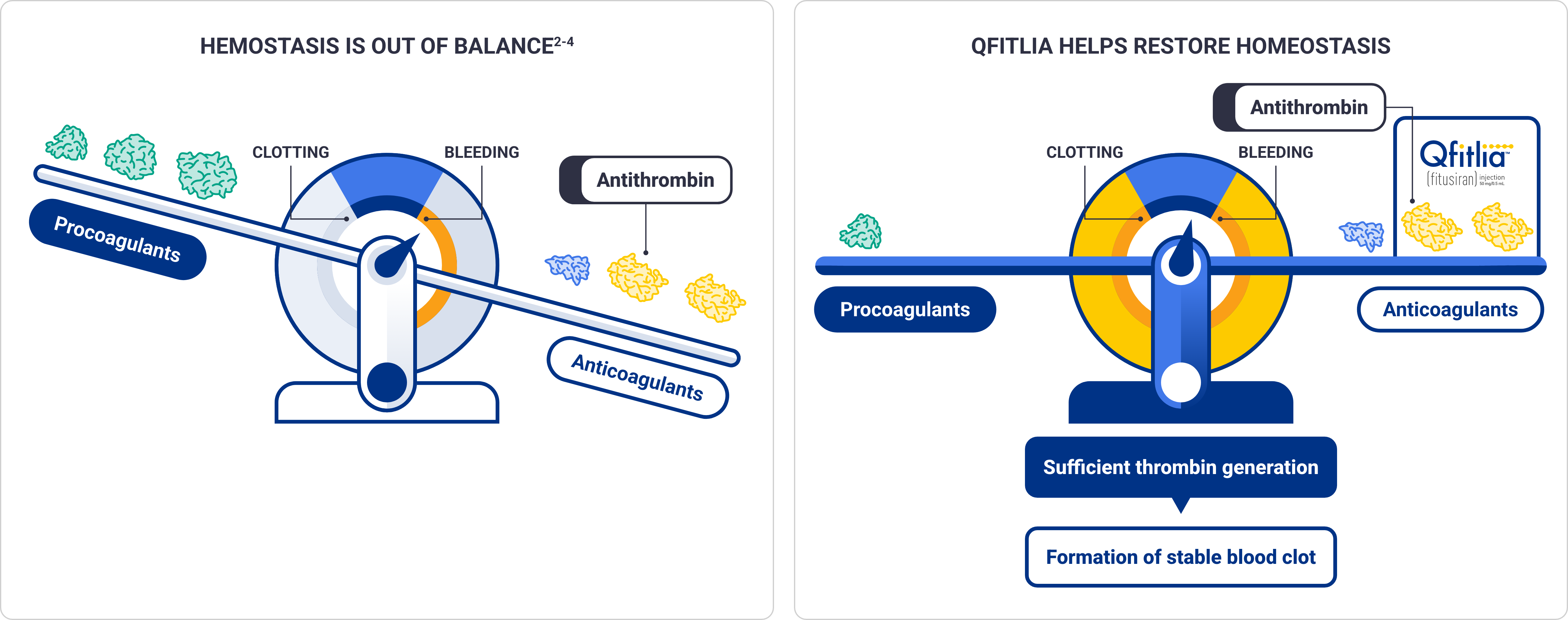
Qfitlia works in a novel way to help restore hemostasis by lowering antithrombin, an inhibitor of thrombin1,2
Lower antithrombin activity levels are associated with lower ABRs; persistent antithrombin activity (<15%) is a risk factor for thrombotic events1*
*The monotonic increasing relationship between antithrombin and ABR was confirmed by modeling and simulation.
ABR=annualized bleed rate; siRNA=small interfering ribonucleic acid.
Breakthrough bleeds should be managed with reduced dose and frequency of
factor or BPA1
Initially, the weight-based dose of factor or BPA should be reduced, and the dosing interval doubled compared with the routine dose
Prophylaxis with Qfitlia leads to increased thrombin generation with additive increase in peak thrombin when used concomitantly with CFC or BPA

Adherence to the bleed management guidelines on the recommended dose of factor replacement or BPA may help minimize the risk of thrombotic events while on Qfitlia

In clinical studies, patients with hemophilia A or B with or without inhibitors underwent major (N=60) and minor (N=71) surgical procedures without discontinuing prophylaxis with Qfitlia
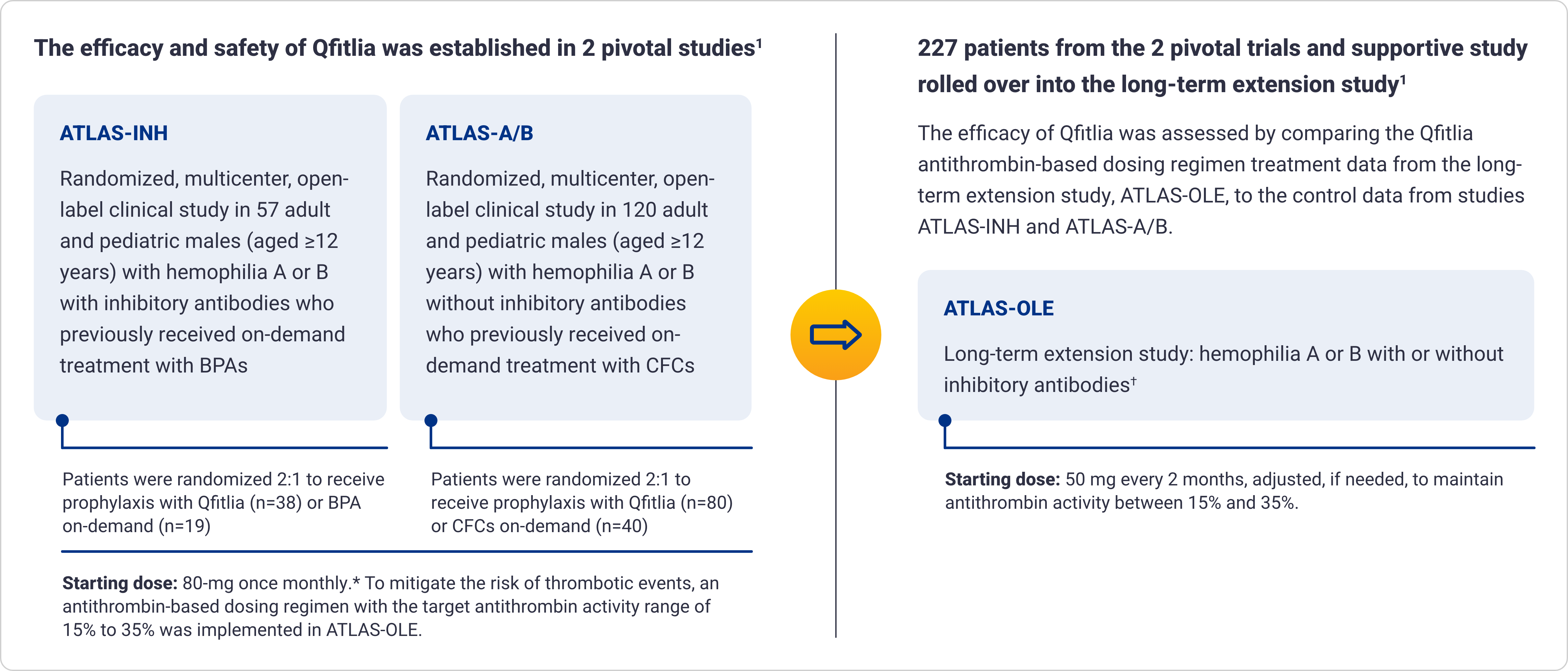
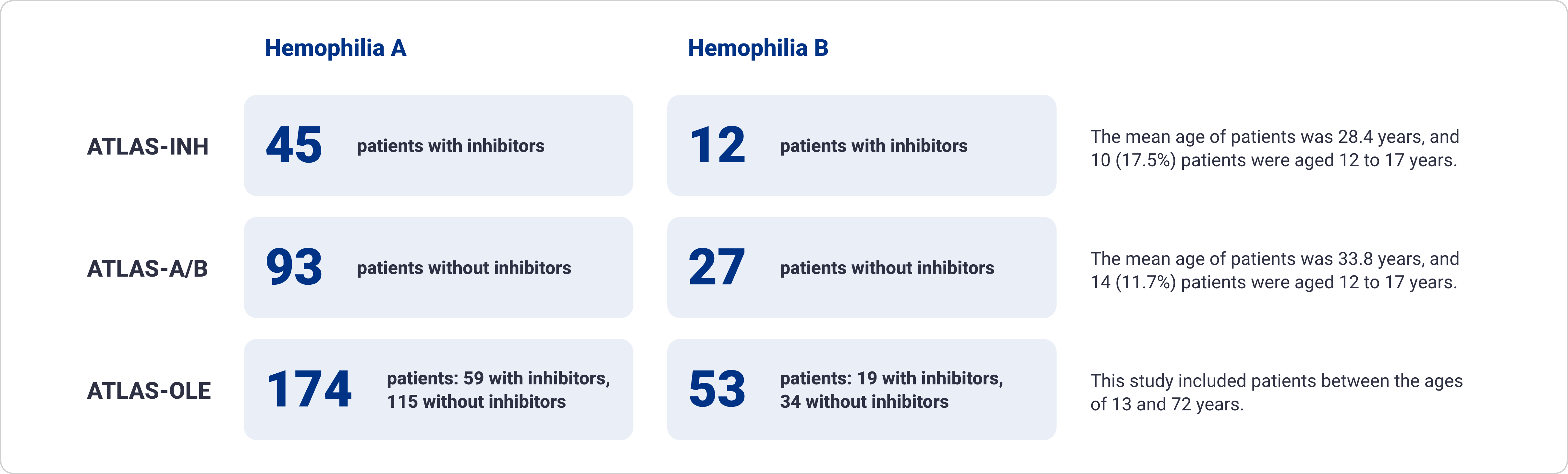
Qfitlia has been extensively studied in patients with hemophilia A and B, with and without inhibitors1-5
Proven bleed protection with as few as 6 injections a year1*
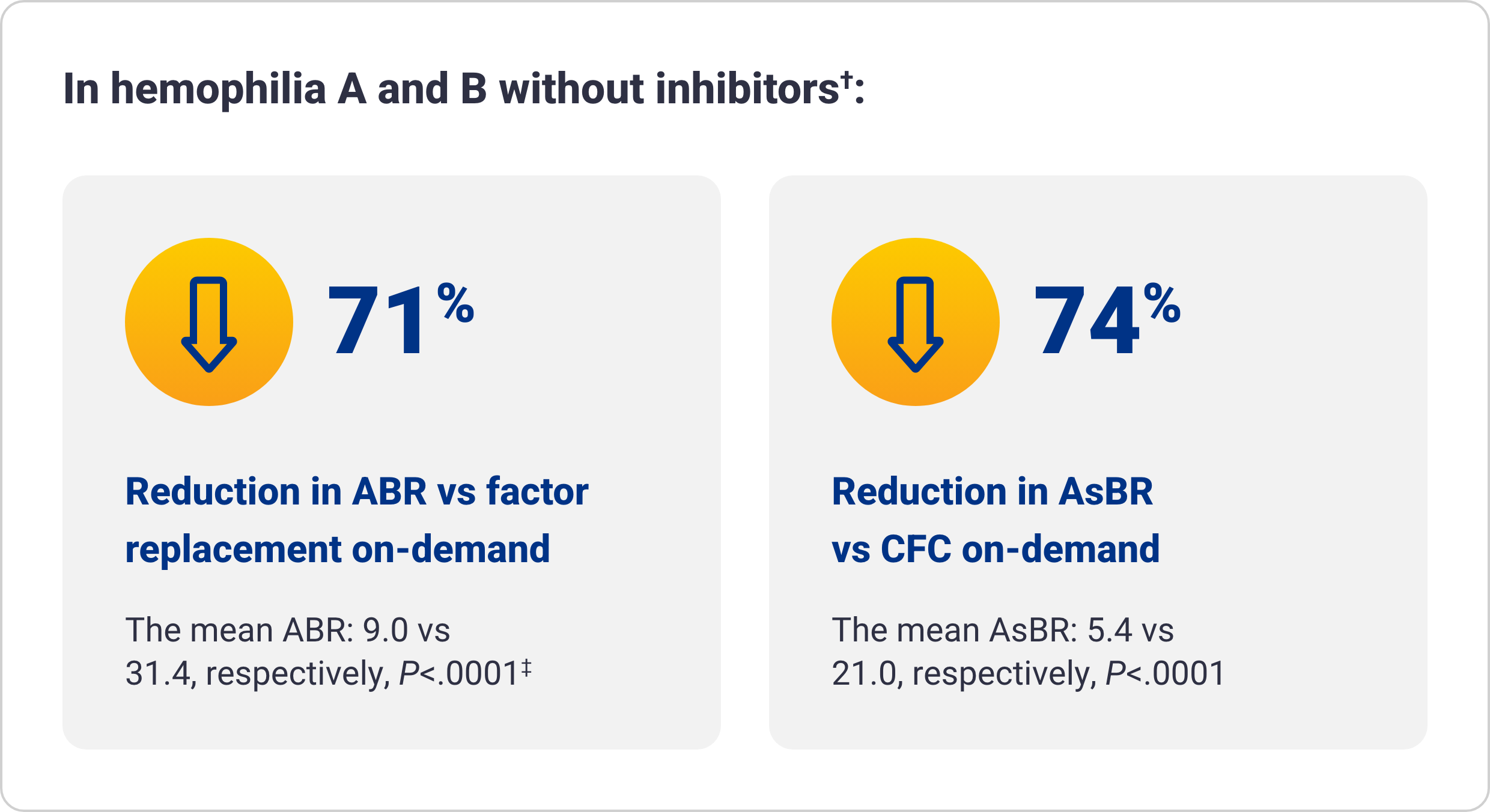
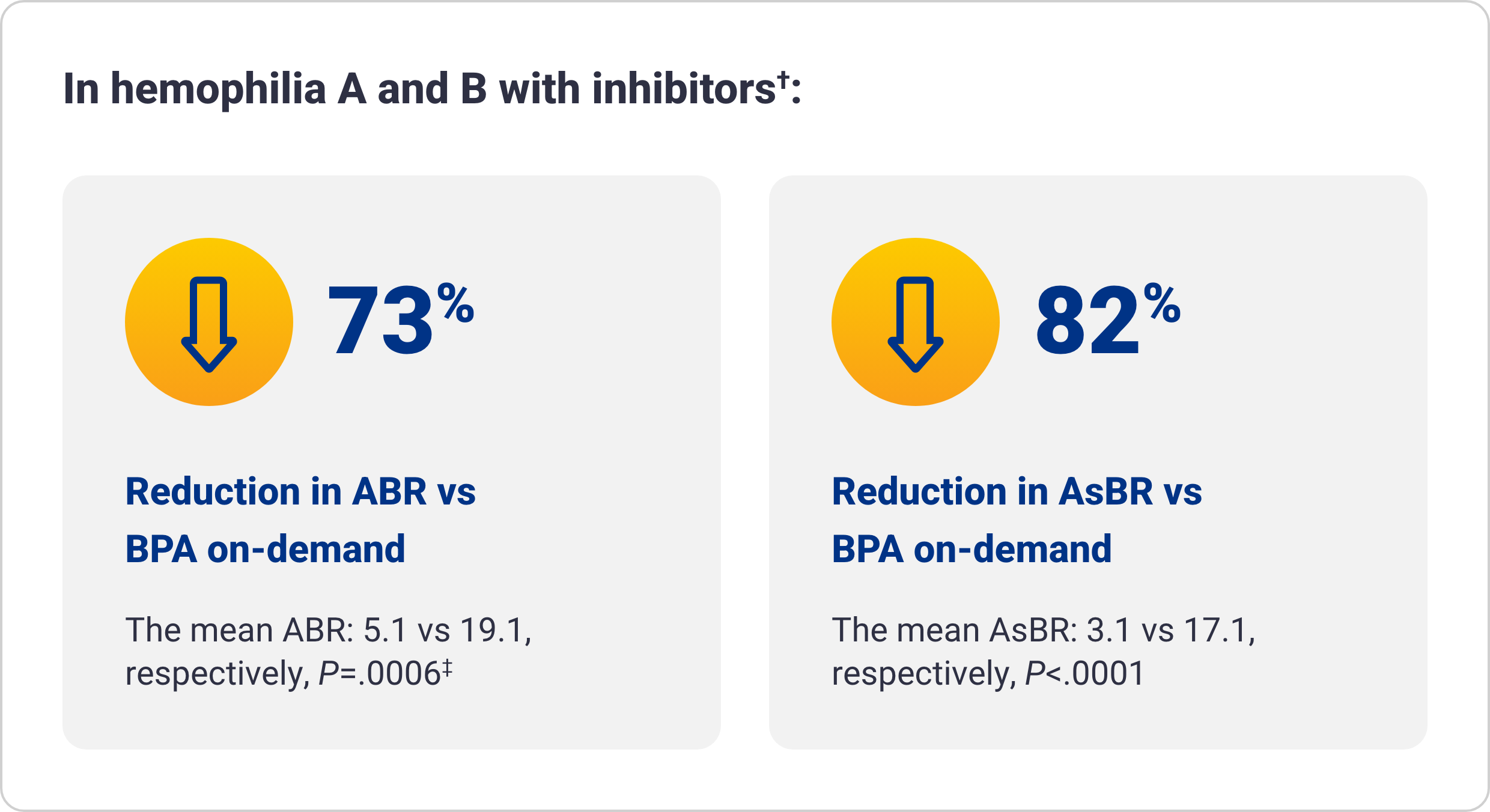
Qfitlia vs on-demand therapies
*In a long-term extension study of 227 patients, ~67% of patients with hemophilia took Qfitlia every other month, and ~19% took it monthly.1
†The integrated efficacy analyses were conducted according to ITT principle, preserving the randomization in the parent studies. The efficacy of Qfitlia was evaluated in 177 patients, comparing Qfitlia prophylaxis under the antithrombin-based dosing regimen in ATLAS-OLE and BPA or factor replacement on-demand in the parent studies.1
‡Based on treated bleeds.
AsBR=annualized spontaneous bleed rate; CFC=clotting factor concentrate; ITT=intent to treat.
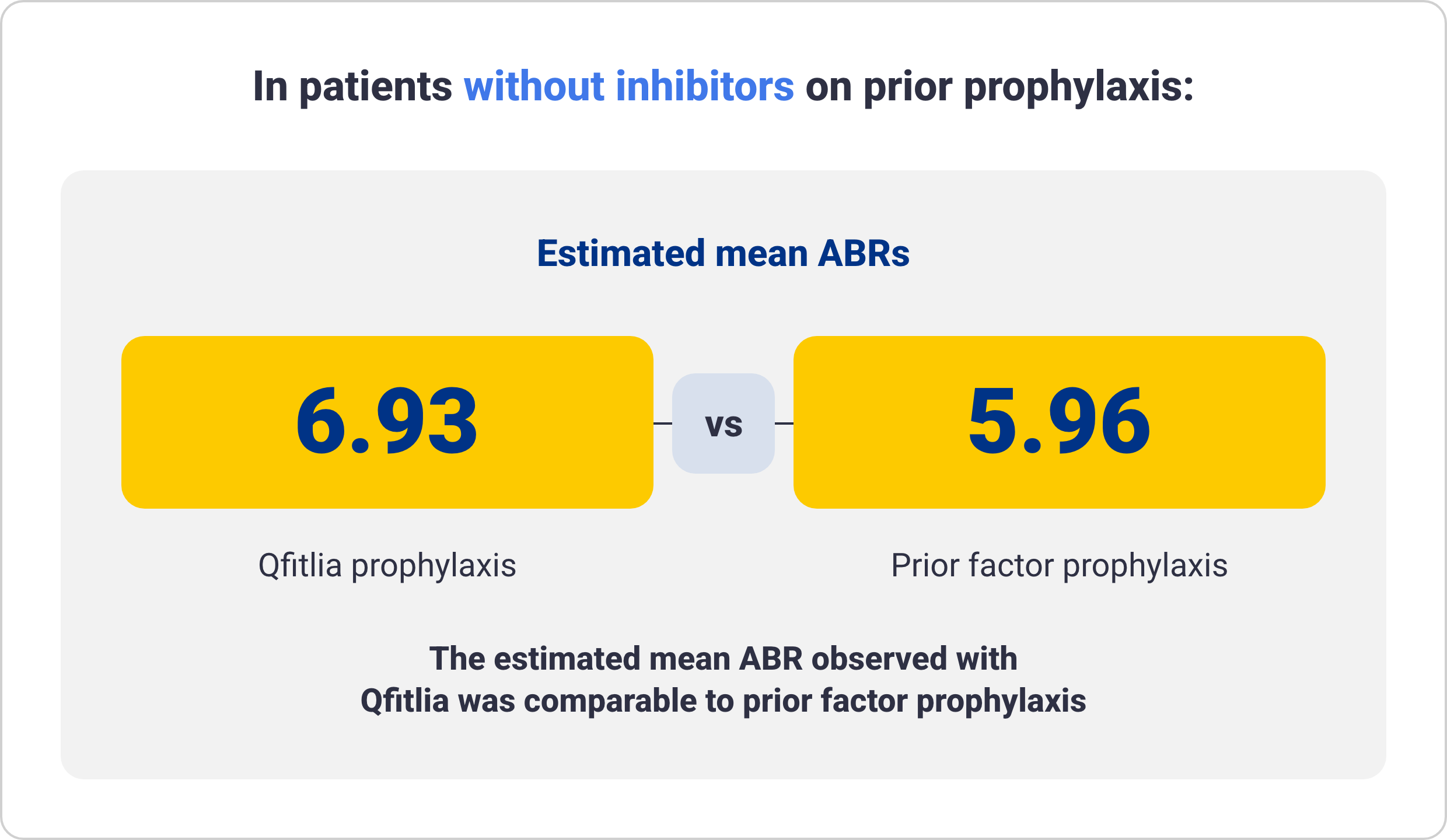
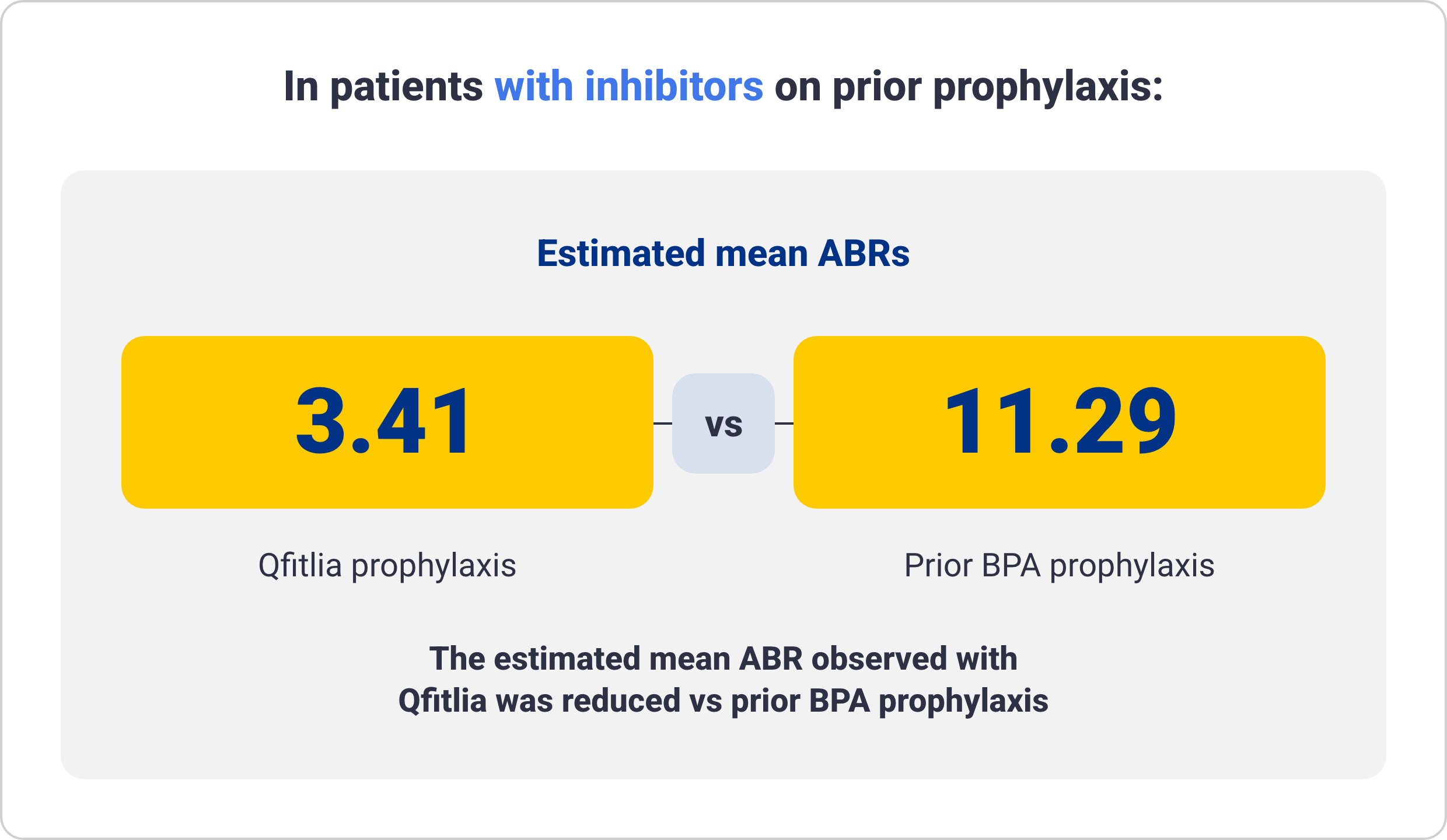
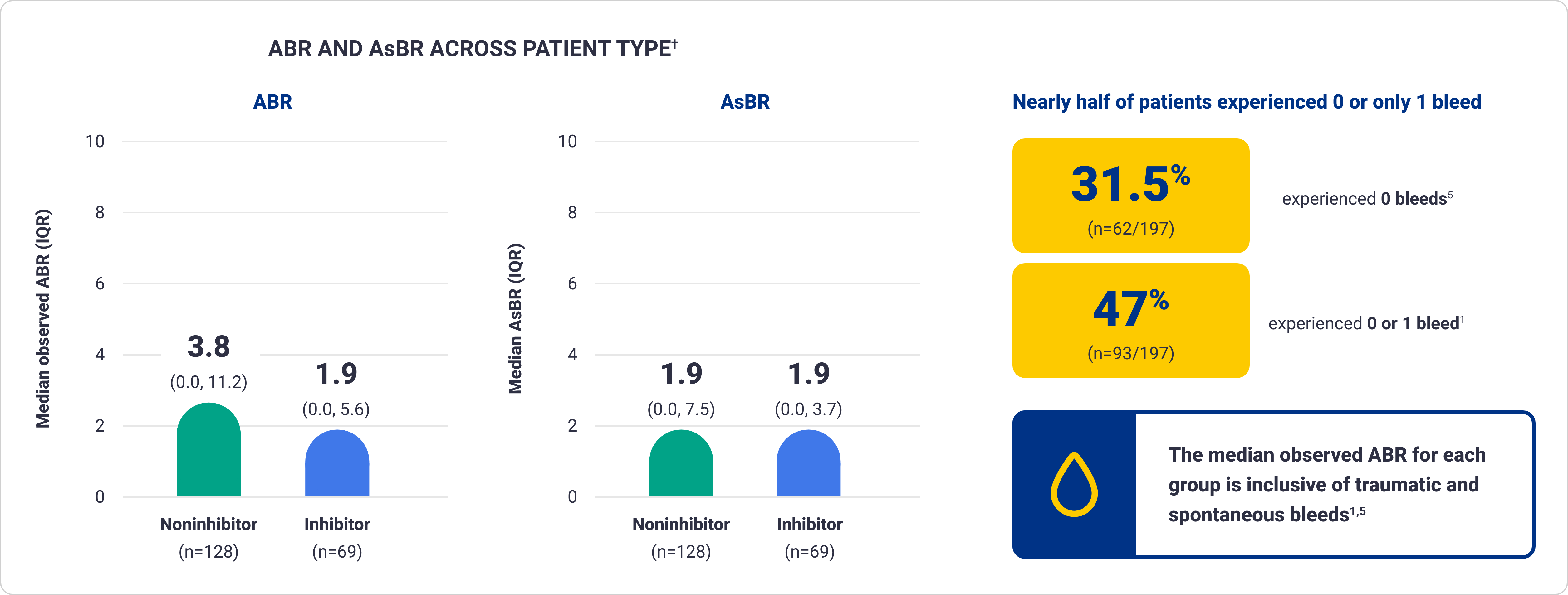
ABR for Qfitlia following prior administration of prophylaxis treatment in patients with hemophilia A and B6
These data are exploratory in nature and not statistically proven. Results are descriptive and definitive conclusions cannot be made.
Data from 69 patients treated with Qfitlia prophylaxis under the antithrombin-based dosing regimen in ATLAS-OLE compared with their prior treatment with BPA or factor
prophylaxis during the onset period in the ATLAS-PPX study.
Bleed rates of Qfitlia observed across subgroups in ATLAS-OLE1*
AsBR and bleed occurrence data are exploratory in nature and not statistically proven. Results are descriptive and definitive conclusions cannot be made.
*ATLAS-OLE is a multicenter, open-label extension study of 227 patients with hemophilia A and B, with or without inhibitors, to evaluate the long-term safety and efficacy of Qfitlia.1
†Based on treated bleeds.
IQR=interquartile range.
Qfitlia has an established safety profile1,5
The safety of Qfitlia has been extensively studied in the largest hemophilia preapproval clinical program7
ADVERSE REACTIONS REPORTED IN ≥5% OF PATIENTS TREATED WITH THE ANTITHROMBIN-BASED DOSING REGIMEN OF QFITLIA (N=286)
| ADVERSE REACTION | % of patients |
|---|---|
| Viral infection* | 29 |
| Nasopharyngitis* | 26 |
| Bacterial infection* | 11 |
| Hepatic injury† | 8 |
| Arthralgia* | 8 |
| Prothrombin fragment 1.2 increased | 7 |
| Injection site reaction* | 6 |
| Headache | 5 |
| Cough* | 5 |
Serious adverse reactions occurred in 4 of 286 (1.4%) patients on the antithrombin-based dosing regimen, 2 of whom had serious adverse reactions of cholecystitis1
Permanent discontinuation of Qfitlia due to an adverse reaction occurred in 4 of 286 (1.4%) patients receiving the antithrombin-based dosing regimen and included liver injury, postoperative deep vein thrombosis, cerebral infarction, and pruritus1
Dosage interruptions of Qfitlia due to an adverse reaction occurred in 2 of 286 (0.7%) patients receiving the antithrombin-based dosing regimen and included increased serum transaminases1
Clinically relevant adverse reactions in <5% of patients include dyspepsia and abdominal pain.
*Includes similar terms.
†Hepatic injury includes alanine aminotransferase increased, aspartate aminotransferase increased, liver injury.
‡Includes injection site bruising, injection site erythema, injection site pain, injection site hematoma, injection site atrophy, injection site hemorrhage, injection site discomfort, injection site swelling, injection site discoloration, injection site pruritus, injection site induration, injection site nodule, injection site mass, injection site vesicles, injection site deformation, injection site rash, injection site joint pain, and application site erythema.
Thrombotic events reported in ATLAS pivotal trial
Thrombotic events were reported in 7 patients (2.6%) receiving fitusiran 80 mg as once-monthly prophylaxis, including 1 fatal event, and 4 patients (1.4%) receiving prophylaxis
with Qfitlia under the antithrombin-based dose regimen.1* Participants with established thrombophilic conditions or a prior history of thrombosis were generally excluded from
studies with Qfitlia.1
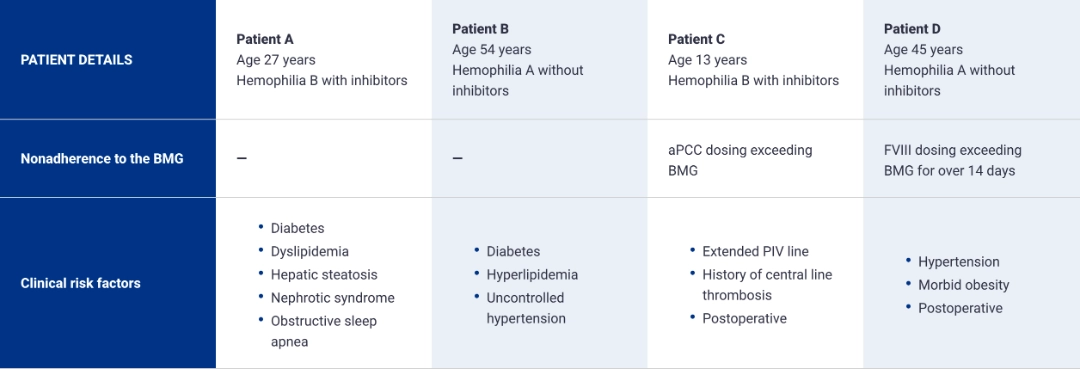
PATIENTS WHO EXPERIENCED THROMBOTIC EVENTS UNDER THE ANTITHROMBIN-BASED DOSING REGIMEN HAD MULTIPLE RISK FACTORS1-8
Risk factors for thrombotic events1
- Antithrombin levels persistently below 15%
- Comorbidities that predispose to thrombosis
- Nonadherence to the BMG in the postoperative setting
- Indwelling venous catheter
- Use of the 80-mg once-monthly dose
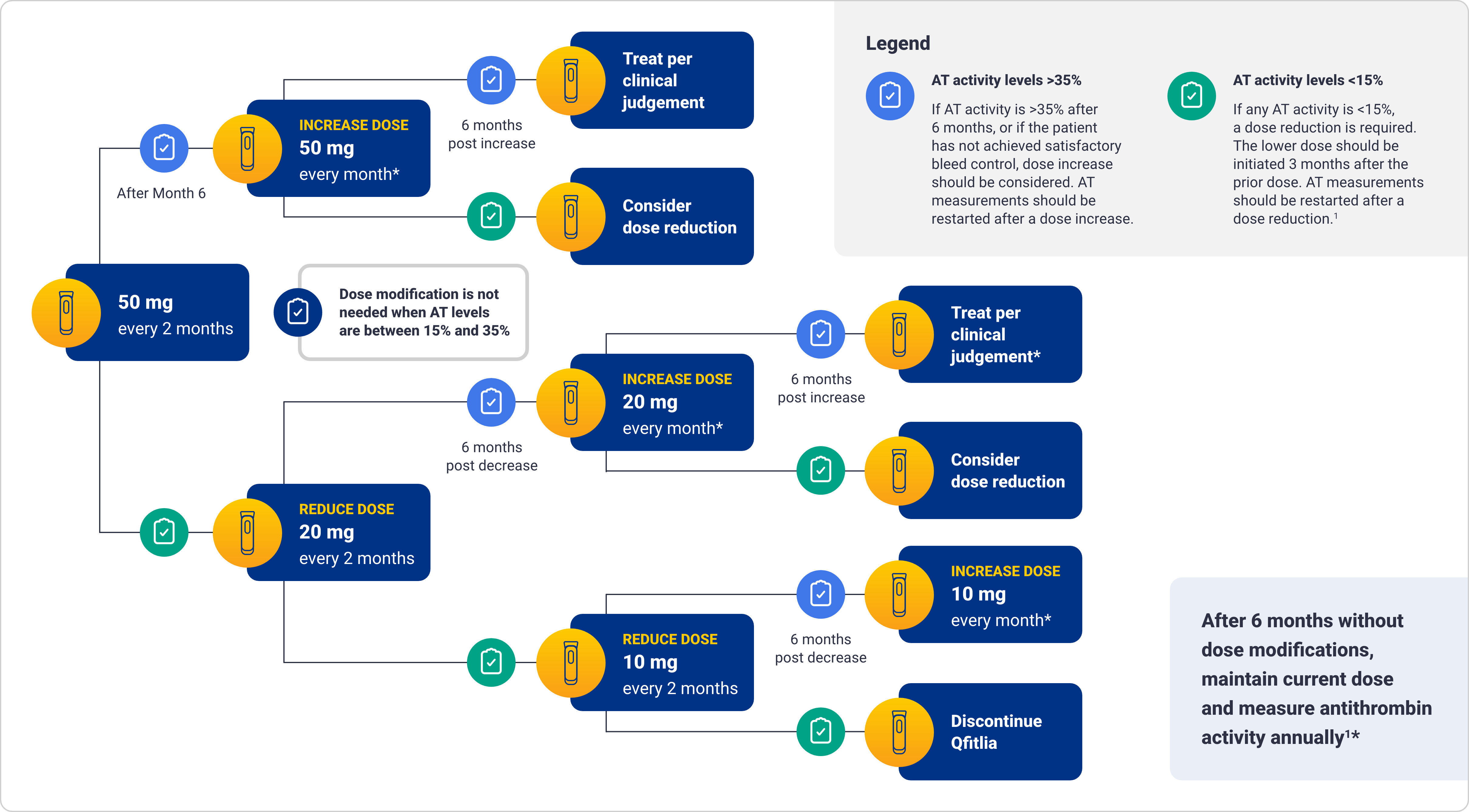
Mitigating thrombotic events risk
- Antithrombin levels should be monitored using an FDA-cleared test and target antithrombin activity in the range of 15% to 35%
- Qfitlia should be used with caution in patients with underlying conditions that may predispose them to a higher risk of thrombosis
- Patients treated with Qfitlia should follow the BMG and be monitored for signs and symptoms of thrombotic events
*The 80-mg once-monthly dosing is not approved or recommended for use.
aPCC=activated prothrombin complex concentrate; BMG=Bleed Management Guidelines; FVIII=Factor VIII; PIV=peripheral intravenous catheter.
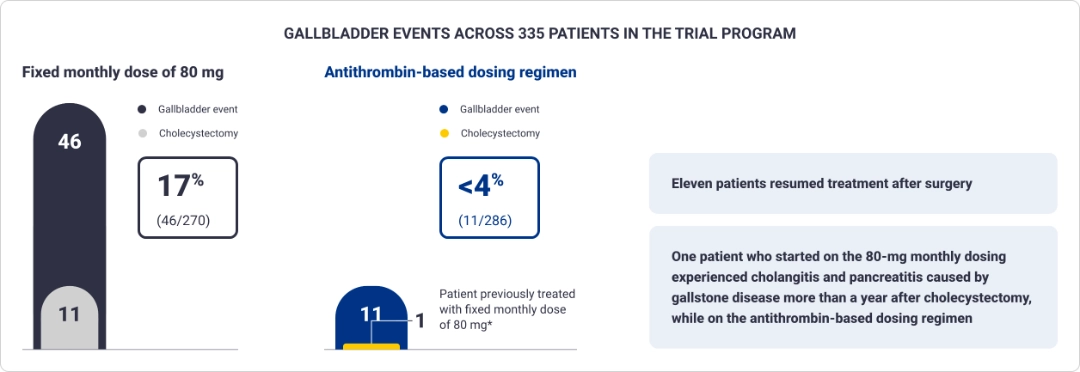
Gallbladder events reported in the ATLAS clinical trial program1
Treatment with Qfitlia is associated with an increased occurrence of acute and recurrent gallbladder disease, including cholelithiasis and cholecystitis. The 80-mg monthly
dosing is not approved or recommended for use.
*Cholecystectomy occurred while patient was on the antithrombin-based dosing regimen.1
Monitoring
Patients should be monitored for signs and symptoms of acute and recurrent gallbladder disease
- Patients diagnosed with acute or recurrent gallbladder disease in the clinical program most commonly presented with symptoms of epigastric pain, generalized abdominal pain, indigestion, nausea, and/or vomiting
- Interrupt or discontinue Qfitlia if acute or recurrent gallbladder disease occurs
- Consider alternative treatment for patients with a history of symptomatic gallbladder disease
Baseline liver function tests should be obtained and monitored monthly for at least 6 months following treatment initiation and dose increases, and periodically thereafter
- On the antithrombin-based dosing regimen, 3.4% of patients treated with Qfitlia had at least 1 alanine aminotransferase value >3x the upper limit of normal (ULN) with a median onset of 89 days after initial dosing (range: 15-768 days)
- Dosage interruptions due to increased transaminases occurred in 2 of 286 (0.7%) patients
- Avoid use of Qfitlia in patients with established hepatic impairment (Child-Pugh Class A, B, and C)
- Interrupt treatment with Qfitlia if alanine aminotransferase or aspartate aminotransferase elevations >5x ULN occur

The value of Qfitlia
Explore how Qfitlia offers prophylactic treatment with the fewest doses for your members.

Connect with us
Schedule a meeting with a member of the Sanofi Market Access team to learn more about Sanofi products.
References: 1. Qfitlia. Prescribing information. Genzyme Corporation; 2025. 2. Negrier C, Shima M, Hoffman M. The central role of thrombin in bleeding disorders. Blood Rev. 2019;38:100582. doi:10.1016/j.blre.2019.05.006 3. Sidonio RF Jr, Hoffman M, Kenet G, Dargaud Y. Thrombin generation and implications for hemophilia therapies: a narrative review. Res Pract Thromb Haemost. 2022;7(1):100018. doi:10.1016/j.rpth.2022.100018 4. Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(suppl 6):1-158. doi:10.1111/hae.14046 5. Young G, Lenting PJ, Croteau SE, Nolan B, Srivastava A. Antithrombin lowering in hemophilia: a closer look at fitusiran. Res Pract Thromb Haemost. 2023;7(4):100179. doi:10.1016/j.rpth.2023.100179 6. Data on file CSR. SAR439774-LTE15174 - fitusiran. November 2023. 7. Young G, Kavakli K, Klamroth R, et al. Safety and efficacy of the fitusiran revised antithrombin-based dose regimen (AT-DR) in people with haemophilia (PwH) A or B, with or without inhibitors (ATLAS-OLE). Presented at: 17th Annual Congress of the European Association for Haemophilia and Allied Disorders (EAHAD); February 6-9, 2024; Frankfurt, Germany. 8. Helixate. Prescribing information. Bayer HealthCare LLC; 2016.
INDICATION
Qfitlia™ (fitusiran) is an antithrombin (AT)-directed small interfering ribonucleic acid (siRNA) indicated for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients aged 12 years and older with hemophilia A or B with or without Factor VIII or IX inhibitors.
WARNING: THROMBOTIC EVENTS AND ACUTE AND RECURRENT GALLBLADDER DISEASE
THROMBOTIC EVENTS
Serious thrombotic events have occurred in Qfitlia-treated patients with risk factors for thromboembolism including persistent antithrombin (AT) activity less than 15%, use of Qfitlia 80 mg once monthly, presence of indwelling venous catheters, and in the post-operative setting when Bleed Management Guidelines were not followed.
Monitor AT activity using an FDA-cleared test and target AT activity 15-35% to reduce the risk of thrombosis. Monitor patients for signs and symptoms of thrombotic events. Interrupt Qfitlia in patients with a thrombotic event and manage as clinically indicated.
ACUTE AND RECURRENT GALLBLADDER DISEASE
Acute and recurrent gallbladder disease, including cholelithiasis and cholecystitis have occurred in Qfitlia-treated patients, some of whom required cholecystectomy or had complications (e.g., pancreatitis) related to gallbladder disease. Monitor patients for signs and symptoms of acute and recurrent gallbladder disease.
Consider interruption or discontinuation of Qfitlia if gallbladder disease occurs. Consider alternative treatment for hemophilia in patients with a history of symptomatic gallbladder disease.
WARNINGS AND PRECAUTIONS
Thrombotic Events:
- Serious thrombotic events have been reported in Qfitlia-treated patients. Thrombotic events were reported in 2.6% of patients receiving the 80 mg once monthly dose, including a fatal event of cerebral venous sinus thrombosis. The 80 mg once monthly dose is not approved or recommended for use. Thrombotic events were reported in 1.4% of patients receiving Qfitlia prophylaxis using the AT-based dose regimen (AT-DR) that targeted AT activity 15-35%
- The risk of thrombosis is greater in patients with certain risk factors (see Boxed WARNING). Treatment of breakthrough bleeding episodes with clotting factor concentrate (CFC) or bypassing agent (BPA) at a dose greater or more frequent than recommended may also increase thrombotic risk
Acute and Recurrent Gallbladder Disease:
- Treatment with Qfitlia is associated with an increased occurrence of acute and recurrent gallbladder disease, including cholelithiasis and cholecystitis (see Boxed WARNING). In the 270 patients in the clinical studies who received Qfitlia at a fixed dose of 80 mg once monthly, 17% experienced gallbladder events and 4% underwent cholecystectomy. In 286 patients who received the AT-DR, 3.8% experienced gallbladder events and 0.3% underwent cholecystectomy
Hepatotoxicity:
- In the two randomized studies testing Qfitlia 80 mg once monthly, alanine transaminase (ALT) and aspartate transaminase (AST) elevations above 3 times the upper limit of normal (ULN) occurred in 32% of patients with hemophilia with inhibitors and 18% of patients with hemophilia without inhibitors. There was one case of moderate hepatic injury attributable to Qfitlia use. On the AT-DR, 3.4% of patients treated with Qfitlia had at least one ALT value >3x ULN
- Avoid use of Qfitlia in patients with hepatic impairment (Child-Pugh Class A, B, and C)
- Obtain baseline liver tests, including AST, ALT, and total bilirubin, prior to initiating Qfitlia, monthly for at least the first 6 months of Qfitlia use, monthly for at least 6 months after a dose increase, and periodically thereafter as clinically indicated
- If new or worsening liver test abnormalities occur, initiate medical management as appropriate and monitor until they return to baseline. If ALT or AST elevations >5x ULN occur, interrupt Qfitlia treatment. If Qfitlia is restarted and ALT or AST elevations >5x ULN reoccur or the patient experiences jaundice due to hepatotoxicity with other causes of liver test elevation ruled out, permanently discontinue Qfitlia
DRUG INTERACTIONS
Hypercoagulability with Concomitant Use of CFC or BPA: Qfitlia prophylaxis leads to increased thrombin generation with additive increase in peak thrombin when used concomitantly with CFC or BPA.
ADVERSE REACTIONS
Common adverse reactions (incidence ≥10%) are viral infection, nasopharyngitis, and bacterial infection.
Please see Full Prescribing Information, including Boxed WARNING.
Learn more about Sanofi’s commitment to fighting counterfeit drugs.
INDICATION
Qfitlia™ (fitusiran) is an antithrombin (AT)-directed small interfering ribonucleic acid (siRNA) indicated for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients aged 12 years and older with hemophilia A or B with or without Factor VIII or IX inhibitors.
WARNING: THROMBOTIC EVENTS AND ACUTE AND RECURRENT GALLBLADDER DISEASE
THROMBOTIC EVENTS
Serious thrombotic events have occurred in Qfitlia-treated patients with risk factors for thromboembolism including persistent antithrombin (AT) activity less than 15%, use of Qfitlia 80 mg once monthly, presence of indwelling venous catheters, and in the post-operative setting when Bleed Management Guidelines were not followed.
Monitor AT activity using an FDA-cleared test and target AT activity 15-35% to reduce the risk of thrombosis. Monitor patients for signs and symptoms of thrombotic events. Interrupt Qfitlia in patients with a thrombotic event and manage as clinically indicated.
ACUTE AND RECURRENT GALLBLADDER DISEASE
Acute and recurrent gallbladder disease, including cholelithiasis and cholecystitis have occurred in Qfitlia-treated patients, some of whom required cholecystectomy or had complications (e.g., pancreatitis) related to gallbladder disease. Monitor patients for signs and symptoms of acute and recurrent gallbladder disease.
Consider interruption or discontinuation of Qfitlia if gallbladder disease occurs. Consider alternative treatment for hemophilia in patients with a history of symptomatic gallbladder disease.
WARNINGS AND PRECAUTIONS
Thrombotic Events:
- Serious thrombotic events have been reported in Qfitlia-treated patients. Thrombotic events were reported in 2.6% of patients receiving the 80 mg once monthly dose, including a fatal event of cerebral venous sinus thrombosis. The 80 mg once monthly dose is not approved or recommended for use. Thrombotic events were reported in 1.4% of patients receiving Qfitlia prophylaxis using the AT-based dose regimen (AT-DR) that targeted AT activity 15-35%
- The risk of thrombosis is greater in patients with certain risk factors (see Boxed WARNING). Treatment of breakthrough bleeding episodes with clotting factor concentrate (CFC) or bypassing agent (BPA) at a dose greater or more frequent than recommended may also increase thrombotic risk
Acute and Recurrent Gallbladder Disease:
- Treatment with Qfitlia is associated with an increased occurrence of acute and recurrent gallbladder disease, including cholelithiasis and cholecystitis (see Boxed WARNING). In the 270 patients in the clinical studies who received Qfitlia at a fixed dose of 80 mg once monthly, 17% experienced gallbladder events and 4% underwent cholecystectomy. In 286 patients who received the AT-DR, 3.8% experienced gallbladder events and 0.3% underwent cholecystectomy
Hepatotoxicity:
- In the two randomized studies testing Qfitlia 80 mg once monthly, alanine transaminase (ALT) and aspartate transaminase (AST) elevations above 3 times the upper limit of normal (ULN) occurred in 32% of patients with hemophilia with inhibitors and 18% of patients with hemophilia without inhibitors. There was one case of moderate hepatic injury attributable to Qfitlia use. On the AT-DR, 3.4% of patients treated with Qfitlia had at least one ALT value >3x ULN
- Avoid use of Qfitlia in patients with hepatic impairment (Child-Pugh Class A, B, and C)
- Obtain baseline liver tests, including AST, ALT, and total bilirubin, prior to initiating Qfitlia, monthly for at least the first 6 months of Qfitlia use, monthly for at least 6 months after a dose increase, and periodically thereafter as clinically indicated
- If new or worsening liver test abnormalities occur, initiate medical management as appropriate and monitor until they return to baseline. If ALT or AST elevations >5x ULN occur, interrupt Qfitlia treatment. If Qfitlia is restarted and ALT or AST elevations >5x ULN reoccur or the patient experiences jaundice due to hepatotoxicity with other causes of liver test elevation ruled out, permanently discontinue Qfitlia
DRUG INTERACTIONS
Hypercoagulability with Concomitant Use of CFC or BPA: Qfitlia prophylaxis leads to increased thrombin generation with additive increase in peak thrombin when used concomitantly with CFC or BPA.
ADVERSE REACTIONS
Common adverse reactions (incidence ≥10%) are viral infection, nasopharyngitis, and bacterial infection.
Please see Full Prescribing Information, including Boxed WARNING.
Learn more about Sanofi’s commitment to fighting counterfeit drugs.
INDICATION
Qfitlia™ (fitusiran) is an antithrombin (AT)-directed small interfering ribonucleic acid (siRNA) indicated for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients aged 12 years and older with hemophilia A or B with or without Factor VIII or IX inhibitors.
WARNING: THROMBOTIC EVENTS AND ACUTE AND RECURRENT GALLBLADDER DISEASE
THROMBOTIC EVENTS
Serious thrombotic events have occurred in Qfitlia-treated patients with risk factors for thromboembolism including persistent antithrombin (AT) activity less than 15%, use of Qfitlia 80 mg once monthly, presence of indwelling venous catheters, and in the post-operative setting when Bleed Management Guidelines were not followed.
Monitor AT activity using an FDA-cleared test and target AT activity 15-35% to reduce the risk of thrombosis. Monitor patients for signs and symptoms of thrombotic events. Interrupt Qfitlia in patients with a thrombotic event and manage as clinically indicated.
ACUTE AND RECURRENT GALLBLADDER DISEASE
Acute and recurrent gallbladder disease, including cholelithiasis and cholecystitis have occurred in Qfitlia-treated patients, some of whom required cholecystectomy or had complications (e.g., pancreatitis) related to gallbladder disease. Monitor patients for signs and symptoms of acute and recurrent gallbladder disease.
Consider interruption or discontinuation of Qfitlia if gallbladder disease occurs. Consider alternative treatment for hemophilia in patients with a history of symptomatic gallbladder disease.
WARNINGS AND PRECAUTIONS
Thrombotic Events:
- Serious thrombotic events have been reported in Qfitlia-treated patients. Thrombotic events were reported in 2.6% of patients receiving the 80 mg once monthly dose, including a fatal event of cerebral venous sinus thrombosis. The 80 mg once monthly dose is not approved or recommended for use. Thrombotic events were reported in 1.4% of patients receiving Qfitlia prophylaxis using the AT-based dose regimen (AT-DR) that targeted AT activity 15-35%
- The risk of thrombosis is greater in patients with certain risk factors (see Boxed WARNING). Treatment of breakthrough bleeding episodes with clotting factor concentrate (CFC) or bypassing agent (BPA) at a dose greater or more frequent than recommended may also increase thrombotic risk
Acute and Recurrent Gallbladder Disease:
- Treatment with Qfitlia is associated with an increased occurrence of acute and recurrent gallbladder disease, including cholelithiasis and cholecystitis (see Boxed WARNING). In the 270 patients in the clinical studies who received Qfitlia at a fixed dose of 80 mg once monthly, 17% experienced gallbladder events and 4% underwent cholecystectomy. In 286 patients who received the AT-DR, 3.8% experienced gallbladder events and 0.3% underwent cholecystectomy
Hepatotoxicity:
- In the two randomized studies testing Qfitlia 80 mg once monthly, alanine transaminase (ALT) and aspartate transaminase (AST) elevations above 3 times the upper limit of normal (ULN) occurred in 32% of patients with hemophilia with inhibitors and 18% of patients with hemophilia without inhibitors. There was one case of moderate hepatic injury attributable to Qfitlia use. On the AT-DR, 3.4% of patients treated with Qfitlia had at least one ALT value >3x ULN
- Avoid use of Qfitlia in patients with hepatic impairment (Child-Pugh Class A, B, and C)
- Obtain baseline liver tests, including AST, ALT, and total bilirubin, prior to initiating Qfitlia, monthly for at least the first 6 months of Qfitlia use, monthly for at least 6 months after a dose increase, and periodically thereafter as clinically indicated
- If new or worsening liver test abnormalities occur, initiate medical management as appropriate and monitor until they return to baseline. If ALT or AST elevations >5x ULN occur, interrupt Qfitlia treatment. If Qfitlia is restarted and ALT or AST elevations >5x ULN reoccur or the patient experiences jaundice due to hepatotoxicity with other causes of liver test elevation ruled out, permanently discontinue Qfitlia
DRUG INTERACTIONS
Hypercoagulability with Concomitant Use of CFC or BPA: Qfitlia prophylaxis leads to increased thrombin generation with additive increase in peak thrombin when used concomitantly with CFC or BPA.
ADVERSE REACTIONS
Common adverse reactions (incidence ≥10%) are viral infection, nasopharyngitis, and bacterial infection.
Please see Full Prescribing Information, including Boxed WARNING.
Learn more about Sanofi’s commitment to fighting counterfeit drugs.
This site is intended for US payers only.
© Sanofi. All rights reserved.
Qfitlia, HemAssist, and Sanofi are trademarks of Sanofi or an affiliate.
Qfitlia is sold under license from Alnylam Pharmaceuticals, Inc.