HEMOPHILIA A/B:
UNMET NEEDS
About hemophilia
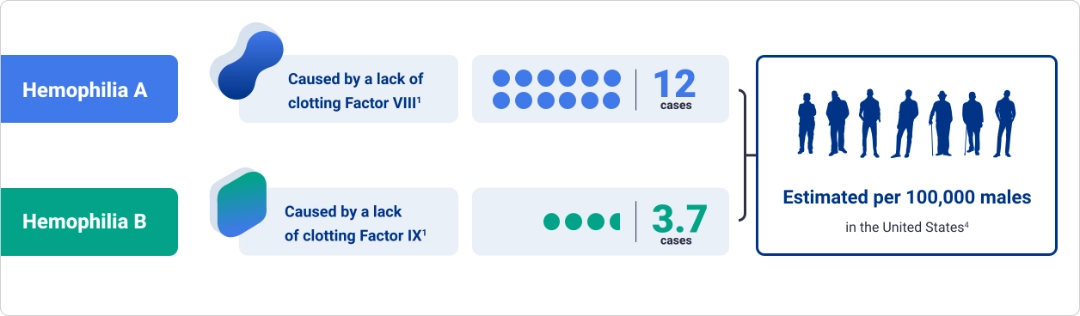
Hemophilia is an inherited bleeding disorder characterized by deficient levels of clotting factor proteins VIII and IX1
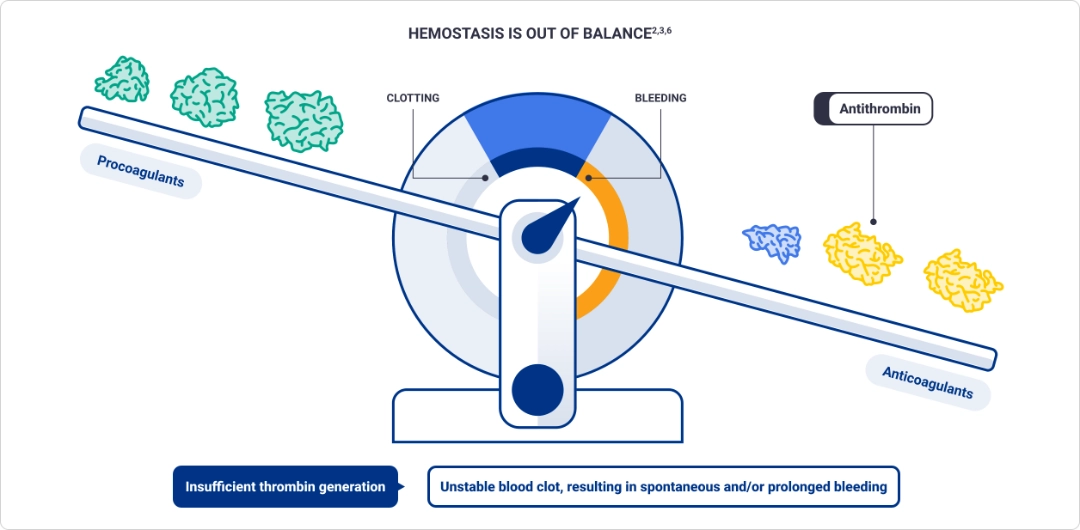
Factor deficiency leads to insufficient thrombin generation, which may cause spontaneous and/or prolonged bleeding2,3
Thrombin is a key procoagulant that helps restore hemostasis2,3
Thrombin converts fibrinogen into fibrin, forming a meshwork that creates a stable blood clot at the site of an injury2
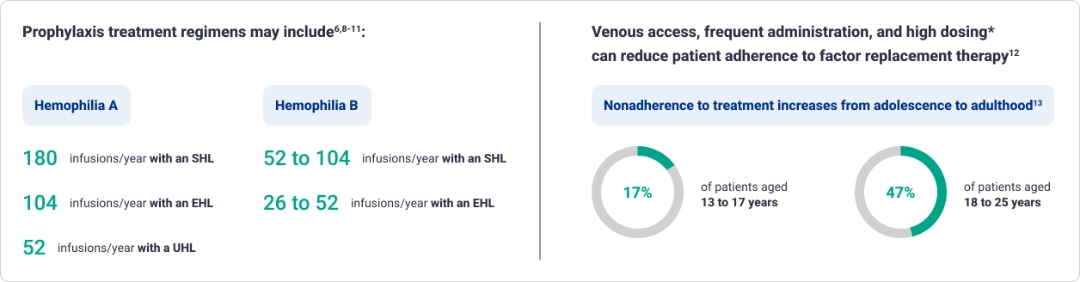
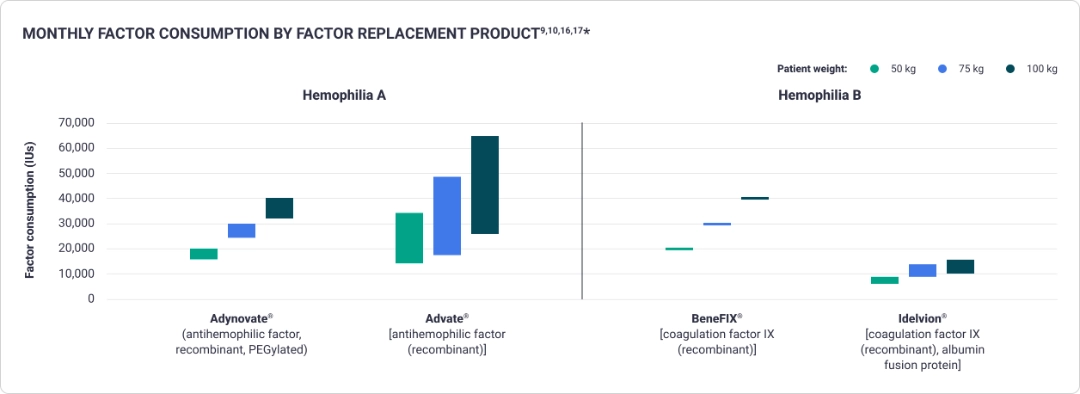
Prophylaxis with factor replacement therapies often requires more frequent dosing6,8,9
The variability of weight-based dosing of factor replacement can result in budget unpredictability9,10,16-18*
Frequent adjustments are made to factor dosing regimens
Dosage and duration of treatment depend on the severity of Factor VIII or IX deficiency, the location and extent of the bleeding, and the patient’s clinical condition.17
- Higher body weight requires a larger dose9,10,16,17*
- Higher factor level targets—based on clinical consensus—may require increased factor doses19
- Higher factor levels result in fewer bleeding episodes5†
*The information on dosing (IU/kg) provided for each product is based on the respective product’s prescribing information.
†Evidence suggests that factor trough levels of 1 to 3 IU/dL (1%-3%) are insufficient to totally prevent bleeds.5
IU/kg=international unit per kilogram.
Treatments for inhibitors have limitations, particularly for hemophilia B6,20-22
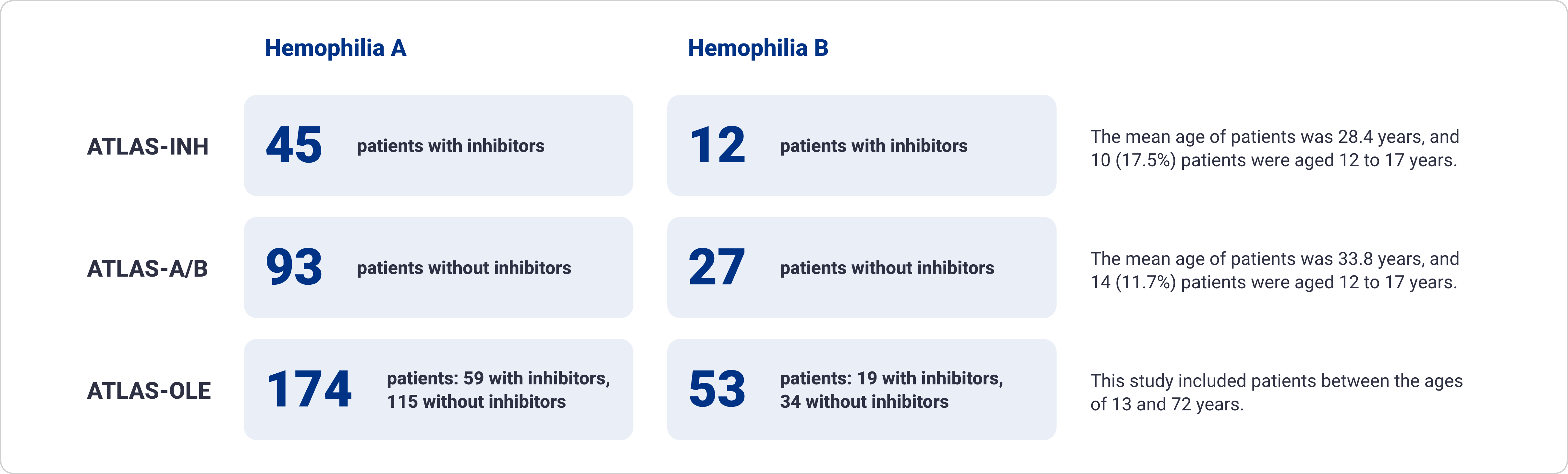
Approximately 30% of patients with hemophilia A and 10% of patients with hemophilia B develop inhibitors6,23
Clinical challenges of patients with inhibitor
Nonfactor agents typically require administration on a daily to weekly basis25,26
Limited approved nonfactor options

In patients with hemophilia B with inhibitors, dosing with an aPCC BPA is typically administered 3 to 5 times weekly and requires a large infusion volume (10 mL/500 units)20‡
Prophylaxis with an rFVIIa BPA may require daily IV infusions27
ITI therapy has limited success
Success rate ≤31% in hemophilia B and 63% to 80% in severe hemophilia A22,28,29
*A low titer inhibitor is a blood test result for a clotting factor inhibitor that is <5 BU/mL.1
†Use of factor therapy for ITI has not been approved by the FDA.
‡A 58-kg patient would need to infuse ~50 mL20
aPCC=activated prothrombin complex concentrate; BPA=bypassing agent; BU=Bethesda unit; FDA=US Food and Drug Administration; FVIII=Factor VIII; ITI=immune tolerance induction; IV=intravenous;
rFVIIa=activated recombinant Factor VII.
Dosing requirements and clinical complications associated with inhibitors result in significant cost impact for payers6,24,30

Patients with inhibitors are twice as likely to be hospitalized for a bleeding complication compared with patients without inhibitors30

Annual costs are 4.8x higher for patients with inhibitors18*†

Patients with inhibitors may be prescribed BPAs, which can cost approximately $2.8 million each year, based on every-other-day prophylaxis dosing of a 70-kg patient31
*Mean annual healthcare expenditures in a population with employer-sponsored insurance were $1,004,731 for patients with inhibitors and $177,711 for patients without inhibitors. In the Medicaid population, expenditures were $644,941 for patients with inhibitors and $154,123 for patients without inhibitors.18
†From a comprehensive and targeted literature review performed in Embase, MEDLINE, and the Cochrane Database of Systematic Reviews covering the period 2010 to 2022 to identify published evidence on medical resource use and healthcare costs (direct and indirect costs) for the treatment of patients with hemophilia A.18


Connect with us
Schedule a meeting with a member of the Sanofi Market Access team to learn more about Sanofi products.
References: 1. About hemophilia. Centers for Disease Control and Prevention. March 5, 2025. Accessed March 7, 2025. https://www.cdc.gov/hemophilia/about/ 2. Sidonio RF Jr, Hoffman M, Kenet G, Dargaud Y. Thrombin generation and implications for hemophilia therapies: a narrative review. Res Pract Thromb Haemost. 2022;7(1):100018. doi:10.1016/j.rpth.2022.100018 3. Negrier C, Shima M, Hoffman M. The central role of thrombin in bleeding disorders. Blood Rev. 2019;38:100582. doi:10.1016/j.blre.2019.05.006 4. Soucie JM, Miller CH, Dupervil B, Le B, Buckner TW. Occurrence rates of haemophilia among males in the United States. Haemophilia. 2020;26(3):487-493. doi:10.1111/hae.13998 5. Community counts: Factor VIII and Factor IX. Centers for Disease Control and Prevention. August 1, 2024. Accessed March 7, 2025. https://www.cdc.gov/hemophilia-community-counts/php/data-research/2024-march-factor-viii-and-factor-ix.html 6. Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for hemophilia. Haemophilia. 2020;26(suppl 6):1-158. doi:10.1111/hae.14046 7. Pasi KJ, Rangarajan S, Georgiev P, et al. RNAi therapy in hemophilia. N Engl J Med. 2017;377(9):819-828. doi:10.1056/NEJMoa1616569 8. Rixubis. Prescribing information. Takeda Pharmaceuticals U.S.A, Inc.; 2025. 9. BeneFIX. Prescribing information. Wyeth Pharmaceuticals LLC; 2022. 10. Advate. Prescribing information. Takeda Pharmaceuticals U.S.A, Inc.; 2023. 11. Altuviiio. Prescribing information. Bioverativ Therapeutics Inc.; 2025. 12. Thornburg CD, Duncan NA. Treatment adherence in hemophilia. Patient Prefer Adher. 2017;11:1677–1686. doi:10.2147/PPA.S139851 13. Martin AP, Burke T, et al. Factor levels & physical activity. Haemophilia. 2020;26(4):711–717. doi:10.1111/hae.13985 14. Mortensen GL, Strand AM, Almén L. Prophylaxis adherence. Haemophilia. 2018;24(6):862–872. doi:10.1111/hae.13621 15. Berntorp E, Hermans C, et al. Optimising prophylaxis. Blood Rev. 2021;50:100852. doi:10.1016/j.blre.2021.100852 16. Adynovate. Prescribing information. Takeda Pharmaceuticals U.S.A, Inc.; 2023. 17. Idelvion. Prescribing information. CSL Behring; 2023. 18. Chen Y, Cheng SJ, et al. Managing hemophilia A costs. J Manag Care Spec Pharm. 2023;29(6):647–658. doi:10.18553/jmcp.2023.29.6.647 19. Miesbach W, von Drygalski A, Smith C, et al. Challenges in hemophilia B. Eur J Haematol. 2024;112(3):339–349. doi:10.1111/ejh.14135 20. Feiba. Prescribing information. Takeda Pharmaceuticals U.S.A, Inc.; 2024. 21. Ellsworth P, Ma A. Factor mimetic therapies. Hematology Am Soc Hematol Educ Program. 2021;2021(1):219–225. doi:10.1182/hematology.2021000253 22. Benson G, Auerswald G, Elezovic I, et al. Immune tolerance induction. Eur J Haematol. 2012;88(5):371–379. doi:10.1111/j.1600-0609.2012.01754.x 23. Male C, Andersson NG, Rafowicz A, et al. PedNet study. Haematologica. 2021;106(1):123–129. doi:10.3324/haematol.2019.239160 24. Shapiro AD, Mitchell IS, Nasr S. Future of bypassing agents. J Thromb Haemost. 2018;16(12):2362–2374. doi:10.1111/jth.14296 25. Alhemo. Prescribing information. Novo Nordisk Inc.; 2025. 26. Hemlibra. Prescribing information. Genentech, Inc.; 2024. 27. Novoeight. Prescribing information. Novo Nordisk A/S; 2020. 28. Malec L, Van Damme A, et al. verITI8 study. Blood. 2023;141(16):1982–1989. doi:10.1182/blood.2022017780 29. Li Z, Sun J, Li Z, et al. Low-dose immune tolerance. Pediatr Investig. 2024;8(2):91–100. doi:10.1002/ped4.12429 30. Oladapo AO, et al. CHESS data burden comparison. Orphanet J Rare Dis. 2018;13(1):198. doi:10.1186/s13023-018-0929-9 31. Pricing comparison for blood disorder treatments. Medicaid Data. Accessed Feb 7, 2025. https://data.medicaid.gov/dataset/...
INDICATION
Qfitlia™ (fitusiran) is an antithrombin (AT)-directed small interfering ribonucleic acid (siRNA) indicated for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients aged 12 years and older with hemophilia A or B with or without Factor VIII or IX inhibitors.
WARNING: THROMBOTIC EVENTS AND ACUTE AND RECURRENT GALLBLADDER DISEASE
THROMBOTIC EVENTS
Serious thrombotic events have occurred in Qfitlia-treated patients with risk factors for thromboembolism including persistent antithrombin (AT) activity less than 15%, use of Qfitlia 80 mg once monthly, presence of indwelling venous catheters, and in the post-operative setting when Bleed Management Guidelines were not followed.
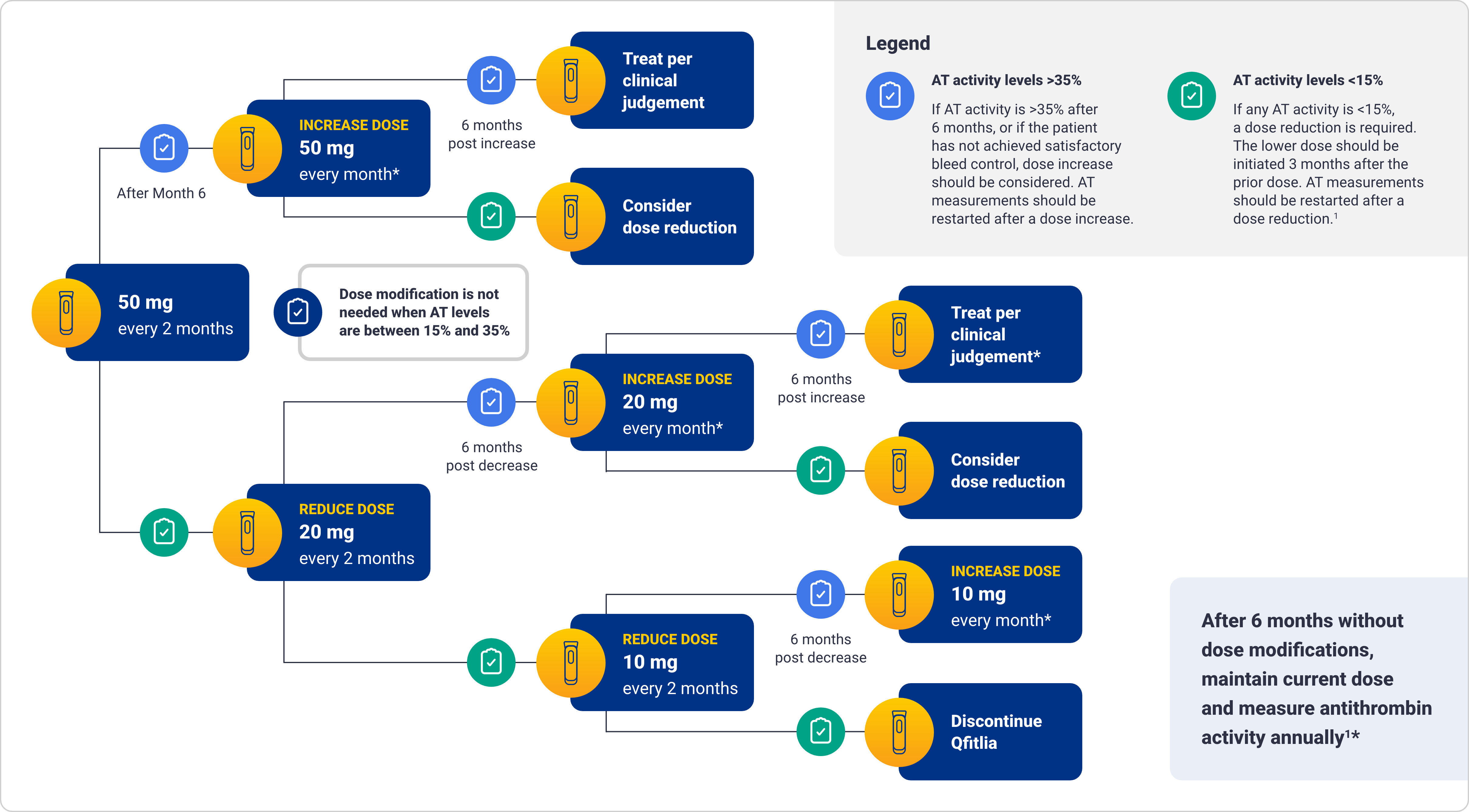
Monitor AT activity using an FDA-cleared test and target AT activity 15-35% to reduce the risk of thrombosis. Monitor patients for signs and symptoms of thrombotic events. Interrupt Qfitlia in patients with a thrombotic event and manage as clinically indicated.
ACUTE AND RECURRENT GALLBLADDER DISEASE
Acute and recurrent gallbladder disease, including cholelithiasis and cholecystitis have occurred in Qfitlia-treated patients, some of whom required cholecystectomy or had complications (e.g., pancreatitis) related to gallbladder disease. Monitor patients for signs and symptoms of acute and recurrent gallbladder disease.
Consider interruption or discontinuation of Qfitlia if gallbladder disease occurs. Consider alternative treatment for hemophilia in patients with a history of symptomatic gallbladder disease.
WARNINGS AND PRECAUTIONS
Thrombotic Events:
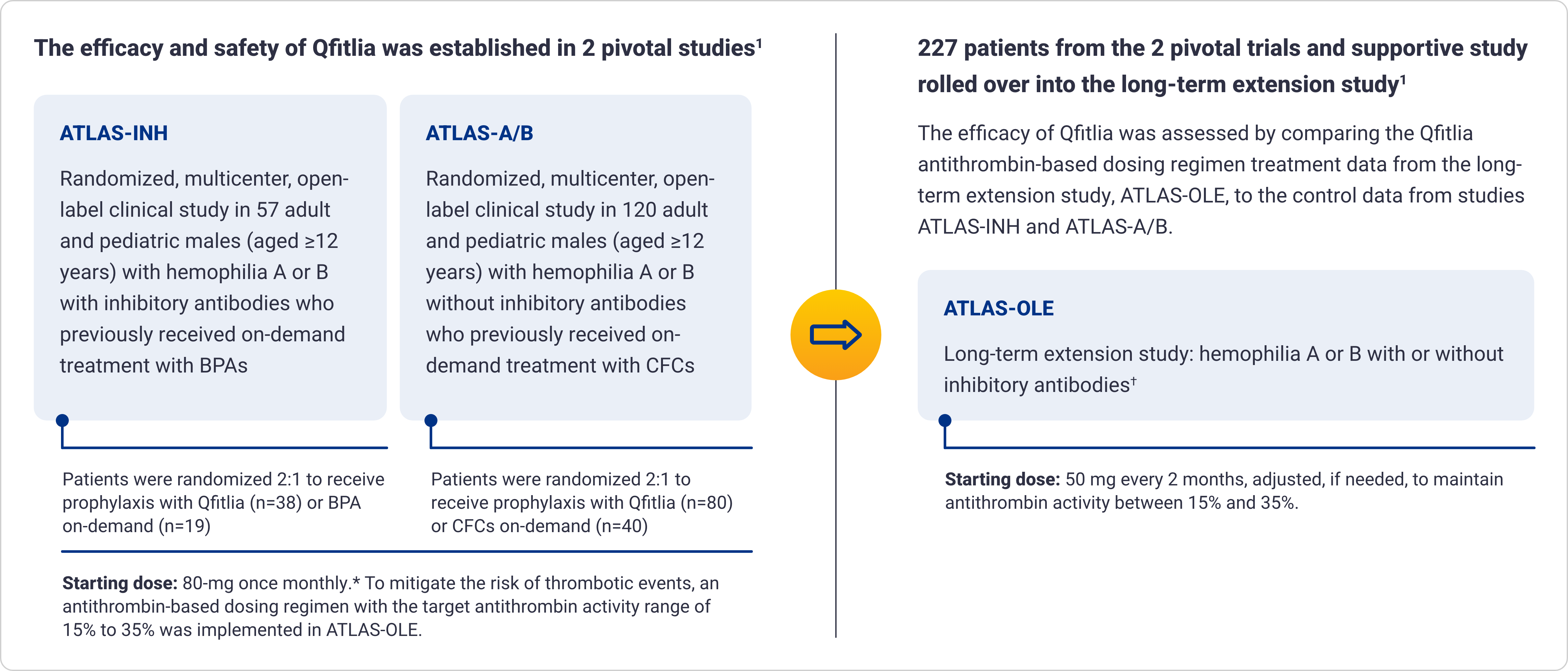
- Serious thrombotic events have been reported in Qfitlia-treated patients. Thrombotic events were reported in 2.6% of patients receiving the 80 mg once monthly dose, including a fatal event of cerebral venous sinus thrombosis. The 80 mg once monthly dose is not approved or recommended for use. Thrombotic events were reported in 1.4% of patients receiving Qfitlia prophylaxis using the AT-based dose regimen (AT-DR) that targeted AT activity 15-35%
- The risk of thrombosis is greater in patients with certain risk factors (see Boxed WARNING). Treatment of breakthrough bleeding episodes with clotting factor concentrate (CFC) or bypassing agent (BPA) at a dose greater or more frequent than recommended may also increase thrombotic risk
Acute and Recurrent Gallbladder Disease:
- Treatment with Qfitlia is associated with an increased occurrence of acute and recurrent gallbladder disease, including cholelithiasis and cholecystitis (see Boxed WARNING). In the 270 patients in the clinical studies who received Qfitlia at a fixed dose of 80 mg once monthly, 17% experienced gallbladder events and 4% underwent cholecystectomy. In 286 patients who received the AT-DR, 3.8% experienced gallbladder events and 0.3% underwent cholecystectomy
Hepatotoxicity:
- In the two randomized studies testing Qfitlia 80 mg once monthly, alanine transaminase (ALT) and aspartate transaminase (AST) elevations above 3 times the upper limit of normal (ULN) occurred in 32% of patients with hemophilia with inhibitors and 18% of patients with hemophilia without inhibitors. There was one case of moderate hepatic injury attributable to Qfitlia use. On the AT-DR, 3.4% of patients treated with Qfitlia had at least one ALT value >3x ULN
- Avoid use of Qfitlia in patients with hepatic impairment (Child-Pugh Class A, B, and C)
- Obtain baseline liver tests, including AST, ALT, and total bilirubin, prior to initiating Qfitlia, monthly for at least the first 6 months of Qfitlia use, monthly for at least 6 months after a dose increase, and periodically thereafter as clinically indicated
- If new or worsening liver test abnormalities occur, initiate medical management as appropriate and monitor until they return to baseline. If ALT or AST elevations >5x ULN occur, interrupt Qfitlia treatment. If Qfitlia is restarted and ALT or AST elevations >5x ULN reoccur or the patient experiences jaundice due to hepatotoxicity with other causes of liver test elevation ruled out, permanently discontinue Qfitlia
DRUG INTERACTIONS
Hypercoagulability with Concomitant Use of CFC or BPA: Qfitlia prophylaxis leads to increased thrombin generation with additive increase in peak thrombin when used concomitantly with CFC or BPA.
ADVERSE REACTIONS
Common adverse reactions (incidence ≥10%) are viral infection, nasopharyngitis, and bacterial infection.
Please see Full Prescribing Information, including Boxed WARNING.
Learn more about Sanofi’s commitment to fighting counterfeit drugs.
INDICATION
Qfitlia™ (fitusiran) is an antithrombin (AT)-directed small interfering ribonucleic acid (siRNA) indicated for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients aged 12 years and older with hemophilia A or B with or without Factor VIII or IX inhibitors.
WARNING: THROMBOTIC EVENTS AND ACUTE AND RECURRENT GALLBLADDER DISEASE
THROMBOTIC EVENTS
Serious thrombotic events have occurred in Qfitlia-treated patients with risk factors for thromboembolism including persistent antithrombin (AT) activity less than 15%, use of Qfitlia 80 mg once monthly, presence of indwelling venous catheters, and in the post-operative setting when Bleed Management Guidelines were not followed.
Monitor AT activity using an FDA-cleared test and target AT activity 15-35% to reduce the risk of thrombosis. Monitor patients for signs and symptoms of thrombotic events. Interrupt Qfitlia in patients with a thrombotic event and manage as clinically indicated.
ACUTE AND RECURRENT GALLBLADDER DISEASE
Acute and recurrent gallbladder disease, including cholelithiasis and cholecystitis have occurred in Qfitlia-treated patients, some of whom required cholecystectomy or had complications (e.g., pancreatitis) related to gallbladder disease. Monitor patients for signs and symptoms of acute and recurrent gallbladder disease.
Consider interruption or discontinuation of Qfitlia if gallbladder disease occurs. Consider alternative treatment for hemophilia in patients with a history of symptomatic gallbladder disease.
WARNINGS AND PRECAUTIONS
Thrombotic Events:
- Serious thrombotic events have been reported in Qfitlia-treated patients. Thrombotic events were reported in 2.6% of patients receiving the 80 mg once monthly dose, including a fatal event of cerebral venous sinus thrombosis. The 80 mg once monthly dose is not approved or recommended for use. Thrombotic events were reported in 1.4% of patients receiving Qfitlia prophylaxis using the AT-based dose regimen (AT-DR) that targeted AT activity 15-35%
- The risk of thrombosis is greater in patients with certain risk factors (see Boxed WARNING). Treatment of breakthrough bleeding episodes with clotting factor concentrate (CFC) or bypassing agent (BPA) at a dose greater or more frequent than recommended may also increase thrombotic risk
Acute and Recurrent Gallbladder Disease:
- Treatment with Qfitlia is associated with an increased occurrence of acute and recurrent gallbladder disease, including cholelithiasis and cholecystitis (see Boxed WARNING). In the 270 patients in the clinical studies who received Qfitlia at a fixed dose of 80 mg once monthly, 17% experienced gallbladder events and 4% underwent cholecystectomy. In 286 patients who received the AT-DR, 3.8% experienced gallbladder events and 0.3% underwent cholecystectomy
Hepatotoxicity:
- In the two randomized studies testing Qfitlia 80 mg once monthly, alanine transaminase (ALT) and aspartate transaminase (AST) elevations above 3 times the upper limit of normal (ULN) occurred in 32% of patients with hemophilia with inhibitors and 18% of patients with hemophilia without inhibitors. There was one case of moderate hepatic injury attributable to Qfitlia use. On the AT-DR, 3.4% of patients treated with Qfitlia had at least one ALT value >3x ULN
- Avoid use of Qfitlia in patients with hepatic impairment (Child-Pugh Class A, B, and C)
- Obtain baseline liver tests, including AST, ALT, and total bilirubin, prior to initiating Qfitlia, monthly for at least the first 6 months of Qfitlia use, monthly for at least 6 months after a dose increase, and periodically thereafter as clinically indicated
- If new or worsening liver test abnormalities occur, initiate medical management as appropriate and monitor until they return to baseline. If ALT or AST elevations >5x ULN occur, interrupt Qfitlia treatment. If Qfitlia is restarted and ALT or AST elevations >5x ULN reoccur or the patient experiences jaundice due to hepatotoxicity with other causes of liver test elevation ruled out, permanently discontinue Qfitlia
DRUG INTERACTIONS
Hypercoagulability with Concomitant Use of CFC or BPA: Qfitlia prophylaxis leads to increased thrombin generation with additive increase in peak thrombin when used concomitantly with CFC or BPA.
ADVERSE REACTIONS
Common adverse reactions (incidence ≥10%) are viral infection, nasopharyngitis, and bacterial infection.
Please see Full Prescribing Information, including Boxed WARNING.
Learn more about Sanofi’s commitment to fighting counterfeit drugs.
INDICATION
Qfitlia™ (fitusiran) is an antithrombin (AT)-directed small interfering ribonucleic acid (siRNA) indicated for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adult and pediatric patients aged 12 years and older with hemophilia A or B with or without Factor VIII or IX inhibitors.
WARNING: THROMBOTIC EVENTS AND ACUTE AND RECURRENT GALLBLADDER DISEASE
THROMBOTIC EVENTS
Serious thrombotic events have occurred in Qfitlia-treated patients with risk factors for thromboembolism including persistent antithrombin (AT) activity less than 15%, use of Qfitlia 80 mg once monthly, presence of indwelling venous catheters, and in the post-operative setting when Bleed Management Guidelines were not followed.
Monitor AT activity using an FDA-cleared test and target AT activity 15-35% to reduce the risk of thrombosis. Monitor patients for signs and symptoms of thrombotic events. Interrupt Qfitlia in patients with a thrombotic event and manage as clinically indicated.
ACUTE AND RECURRENT GALLBLADDER DISEASE
Acute and recurrent gallbladder disease, including cholelithiasis and cholecystitis have occurred in Qfitlia-treated patients, some of whom required cholecystectomy or had complications (e.g., pancreatitis) related to gallbladder disease. Monitor patients for signs and symptoms of acute and recurrent gallbladder disease.
Consider interruption or discontinuation of Qfitlia if gallbladder disease occurs. Consider alternative treatment for hemophilia in patients with a history of symptomatic gallbladder disease.
WARNINGS AND PRECAUTIONS
Thrombotic Events:
- Serious thrombotic events have been reported in Qfitlia-treated patients. Thrombotic events were reported in 2.6% of patients receiving the 80 mg once monthly dose, including a fatal event of cerebral venous sinus thrombosis. The 80 mg once monthly dose is not approved or recommended for use. Thrombotic events were reported in 1.4% of patients receiving Qfitlia prophylaxis using the AT-based dose regimen (AT-DR) that targeted AT activity 15-35%
- The risk of thrombosis is greater in patients with certain risk factors (see Boxed WARNING). Treatment of breakthrough bleeding episodes with clotting factor concentrate (CFC) or bypassing agent (BPA) at a dose greater or more frequent than recommended may also increase thrombotic risk
Acute and Recurrent Gallbladder Disease:
- Treatment with Qfitlia is associated with an increased occurrence of acute and recurrent gallbladder disease, including cholelithiasis and cholecystitis (see Boxed WARNING). In the 270 patients in the clinical studies who received Qfitlia at a fixed dose of 80 mg once monthly, 17% experienced gallbladder events and 4% underwent cholecystectomy. In 286 patients who received the AT-DR, 3.8% experienced gallbladder events and 0.3% underwent cholecystectomy
Hepatotoxicity:
- In the two randomized studies testing Qfitlia 80 mg once monthly, alanine transaminase (ALT) and aspartate transaminase (AST) elevations above 3 times the upper limit of normal (ULN) occurred in 32% of patients with hemophilia with inhibitors and 18% of patients with hemophilia without inhibitors. There was one case of moderate hepatic injury attributable to Qfitlia use. On the AT-DR, 3.4% of patients treated with Qfitlia had at least one ALT value >3x ULN
- Avoid use of Qfitlia in patients with hepatic impairment (Child-Pugh Class A, B, and C)
- Obtain baseline liver tests, including AST, ALT, and total bilirubin, prior to initiating Qfitlia, monthly for at least the first 6 months of Qfitlia use, monthly for at least 6 months after a dose increase, and periodically thereafter as clinically indicated
- If new or worsening liver test abnormalities occur, initiate medical management as appropriate and monitor until they return to baseline. If ALT or AST elevations >5x ULN occur, interrupt Qfitlia treatment. If Qfitlia is restarted and ALT or AST elevations >5x ULN reoccur or the patient experiences jaundice due to hepatotoxicity with other causes of liver test elevation ruled out, permanently discontinue Qfitlia
DRUG INTERACTIONS
Hypercoagulability with Concomitant Use of CFC or BPA: Qfitlia prophylaxis leads to increased thrombin generation with additive increase in peak thrombin when used concomitantly with CFC or BPA.
ADVERSE REACTIONS
Common adverse reactions (incidence ≥10%) are viral infection, nasopharyngitis, and bacterial infection.
Please see Full Prescribing Information, including Boxed WARNING.
Learn more about Sanofi’s commitment to fighting counterfeit drugs.
This site is intended for US payers only.
© Sanofi. All rights reserved.
Qfitlia, HemAssist, and Sanofi are trademarks of Sanofi or an affiliate.
Qfitlia is sold under license from Alnylam Pharmaceuticals, Inc.