WAYRILZ: Clinical evidence
Introducing WAYRILZ (rilzabrutinib), a multi-immune modulator that targets complex immune dysregulation via BTK inhibition
Indication1
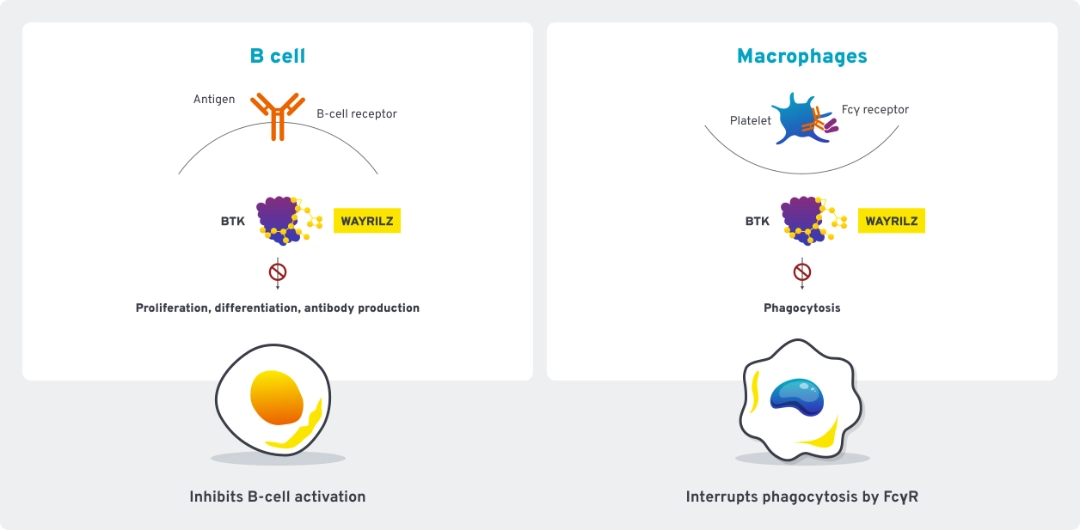
Mechanism of action1,2
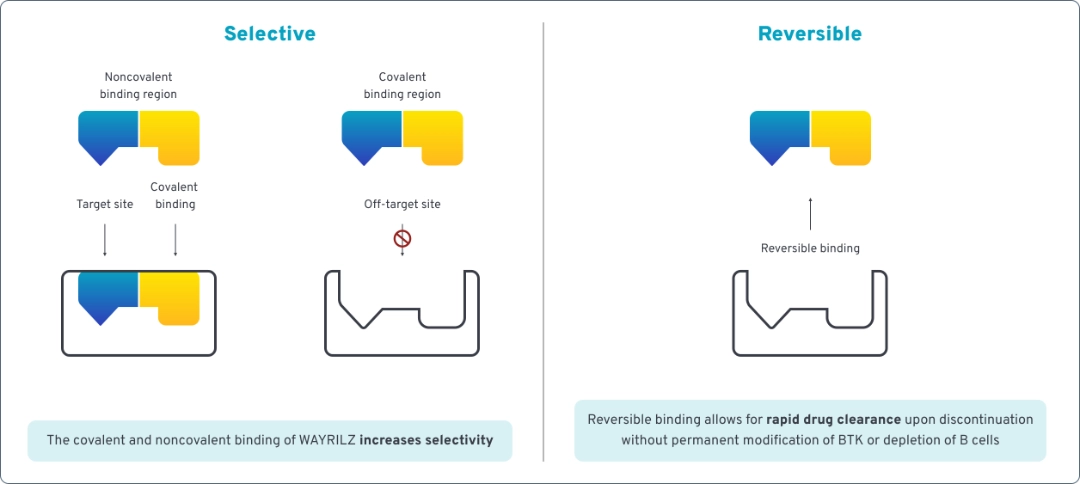
WAYRILZ is a selective, reversible Bruton tyrosine kinase (BTK) inhibitor specifically designed to treat autoimmune dysregulation in ITP. It uses BTK inhibition to modulate multiple immune pathways.
As a next-generation BTK inhibitor, WAYRILZ selectively and reversibly inhibits BTK.1,2
Clinical studies demonstrate that WAYRILZ offers a novel, multifaceted strategy to managing ITP
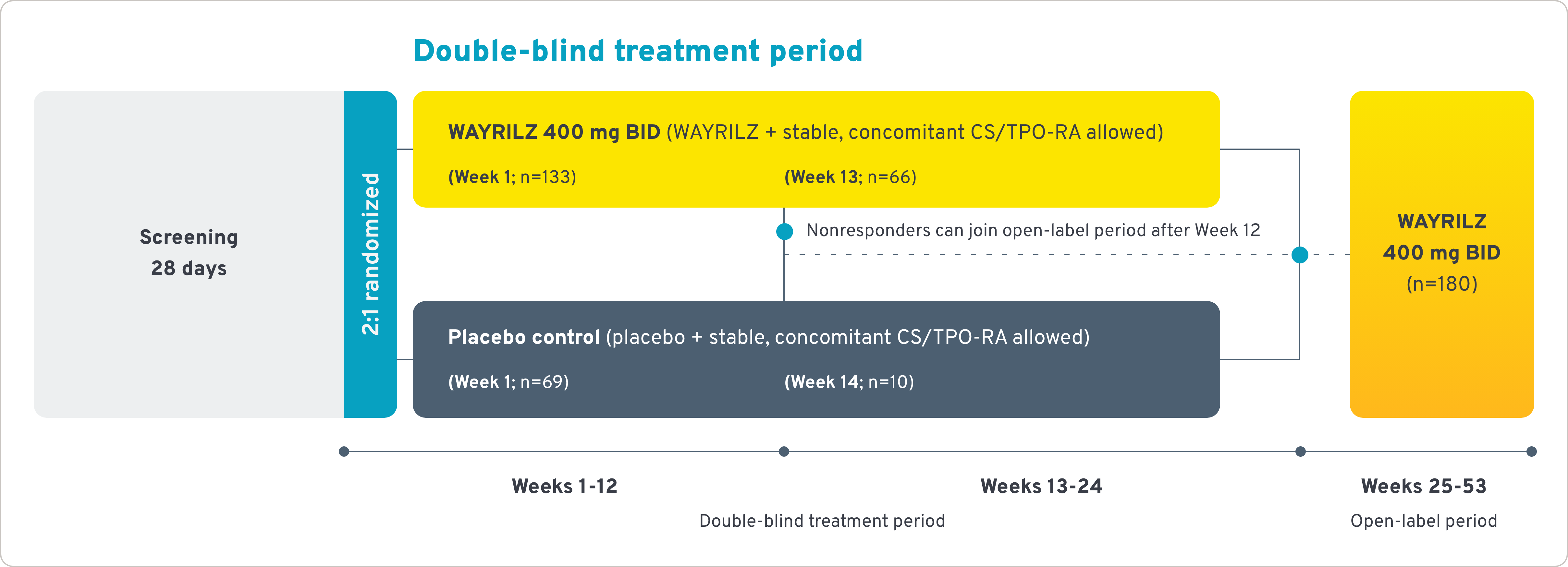
LUNA 3 study
WAYRILZ was studied in a wide range of patients, from second-line to heavily pretreated.1,3
WAYRILZ demonstrated durable and rapid platelet response vs placebo1,2
PRIMARY ENDPOINT
Durable platelet response: A platelet count of >50x109/L for >5 of at least 8 non-missing weekly measurements during the last 12 weeks of the double blind period, including >2 such responses in the last 6 weeks without rescue therapy.1,2
FASTER AND LONGER-LASTING RESPONSE VS PLACEBO CONTROL SHOWN IN ALL PATIENTS TREATED WITH WAYRILZ
|
TIME TO FIRST PLATELET RESPONSEa
|
|
| 36 days | All WAYRILZ arm (n=133) (vs placebo control, P<0.0001) |
| NOT REACHED | Placebo control (n=69) |
| 15 days | WAYRILZ respondersb (n=85) |
|
NUMBER OF WEEKS WITH PLATELET RESPONSEc
|
|
| 7 weeks | All WAYRILZ arm (n=133) (vs placebo control, P<0.0001) |
| <1 week | Placebo control (n=69) |
| 11 weeks | WAYRILZ respondersb (n=85) |
aPlatelet count ≥50 × 109/L and at least doubled from baseline in the absence of rescue therapy.
bResponders had ≥1 platelet response of at least 50 × 109/L or ≥30 × 109/L and at least doubled from baseline by Week 13 without rescue therapy.
cPlatelet count of ≥50 × 109/L or between 30 × 109/L and <50 × 109/L and doubled from baseline. Platelet counts assessed within 4 weeks of rescue medication intake are considered as no response; missing weekly platelet counts due to any reason are considered as no response.
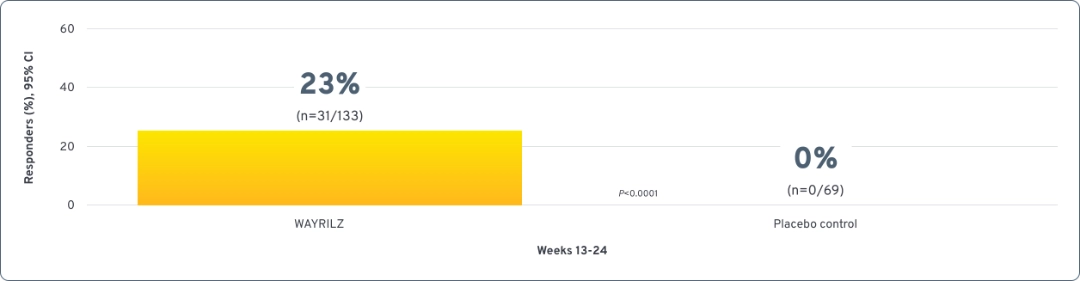
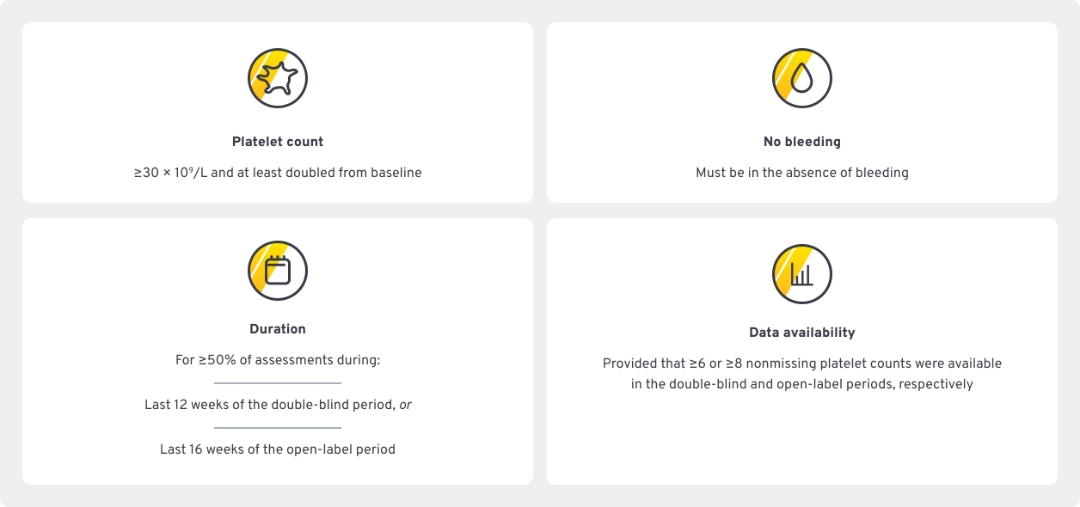
Post-hoc analysis: Modified durable response
Modified durable response4,5
Defined using the international working group (IWG)a Standard for platelet response as part of the criteria:
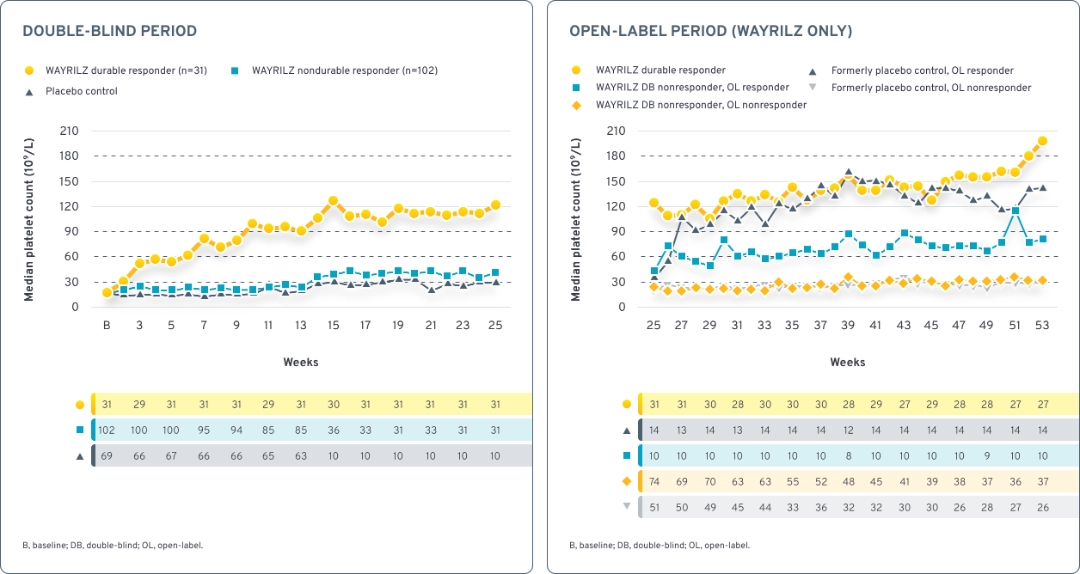
Median platelet counts throughout the double-blind and open-label periods1,3,6
Durable responders achieved median counts exceeding 30 × 109/L, 50 × 109/L, and 100 x 109/L at Weeks 2, 3, and 14, respectively, and maintained >100 × 109/L thereafter. Nondurable responders achieved median counts >30 × 109/L from Weeks 14 to 25. In the open-label period, some WAYRILZ double-blind nondurable responders were able to achieve median platelet counts above 60x109/L.
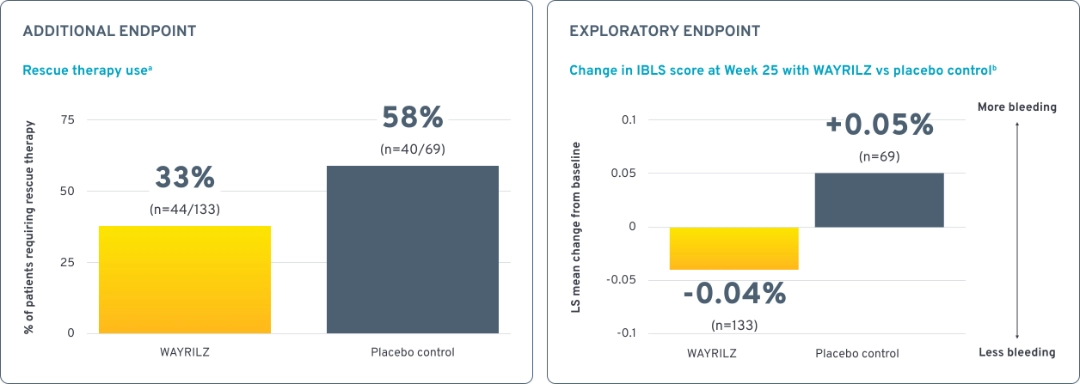
Rescue therapy use and bleeding assessment scores1,2
ESTABLISHED SAFTEY PROFILE IN LUNA 3 WITH WAYRILZ1,3
Common adverse reactions in patients with ITP during the double-blind period of the LUNA 3 studya
|
|
|
|
||
|---|---|---|---|---|
|
|
|
|
|
|
| Diarrhea | 32 | 0 | 10 | 0 |
| Nausea | 20 | 0 | 6 | 0 |
| Headache | 18 | 0 | 7 | 0 |
| Abdominal painb | 14 | 0 | 1 | 0 |
| COVID-19 | 14 | 0.8 | 4 | 0 |
| Arthralgia | 9 | 0 | 4 | 0 |
| Dizziness | 8 | 0 | 1 | 0 |
| Nasopharyngitisb | 7 | 0 | 3 | 0 |
| Vomiting | 7 | 0 | 1 | 0 |
| Dyspepsia | 5 | 0 | 0 | 0 |
| Cough | 5 | 0 | 0 | 0 |
In patients who experience gastrointestinal symptoms, taking WAYRILZ with food may improve tolerability.
AR, adverse reaction; COVID-19, coronavirus disease 2019.
aAdverse reactions that occurred in at least 5% of WAYRILZ-treated patients and at least 3% higher than in placebo-treated patients.
bGrouped term.
WAYRILZ has an established safety profile1,3,6

Serious infections
An increased risk of serious infections (including bacterial, viral, or fungal) can occur in patients treated with BTK inhibitors, including WAYRILZ. In the LUNA 3 trial, fatal pneumonia occurred in one participant in the WAYRILZ group. Other serious infections (1 each, 0.8%) included COVID-19 infection, wound infection, and one patient experienced urinary tract infection and kidney abscess. Monitor patients for signs and symptoms of infection and treat appropriately

Hepatoxicity, including drug-induced liver injury
Hepatoxicity, including severe, life-threatening, and potentially fatal cases of drug-induced liver injury (DILI), can occur in patients treated with BTK inhibitors. Elevations of liver transaminases occurred with WAYRILZ in the ITP clinical trials and were generally mild to moderate in severity. Evaluate bilirubin and transaminases at baseline and as clinically indicated during treatment with WAYRILZ. For patients who develop abnormal liver tests after WAYRILZ, monitor more frequently. If DILI is suspected, withhold WAYRILZ. Upon confirmation of DILI, discontinue WAYRILZ

Embryo-fetal toxicity
Based on findings from preliminary animal reproduction studies, WAYRILZ may cause fetal harm when administered to a pregnant woman. Verify pregnancy status of females of reproductive potential prior to initiating WAYRILZ treatment. Advise females of reproductive potential to use effective contraception while taking WAYRILZ and for 1 week after the final dose

Discontinuations
More patients in the WAYRILZ arm completed the 24-week double-blind period (47%) vs placebo control (14%). The main reason for discontinuation during the overall 24-week double-blind period was lack of platelet response per predefined levels (41% for WAYRILZ vs 80% for placebo control). Adverse reactions resulting in discontinuation of WAYRILZ included erythema nodosum, neutropenia, arthralgia, dyspepsia, headache, pain in extremity, abdominal discomfort, diarrhea, nausea, and pneumonia and occurred in 4.5% of patients

No treatment-related atrial fibrillation or anemia with WAYRILZ
There were no instances of treatment-related atrial fibrillation or anemia

One patient had a TE in the WAYRILZ arm
The patient was on a concomitant TPO-RA and had a history of comorbidities prior to study start

No new safety signals during the open-label period
WAYRILZ product profile
Dosing and administration1

The recommended dosage of
WAYRILZ is 400 mg taken orally
twice daily
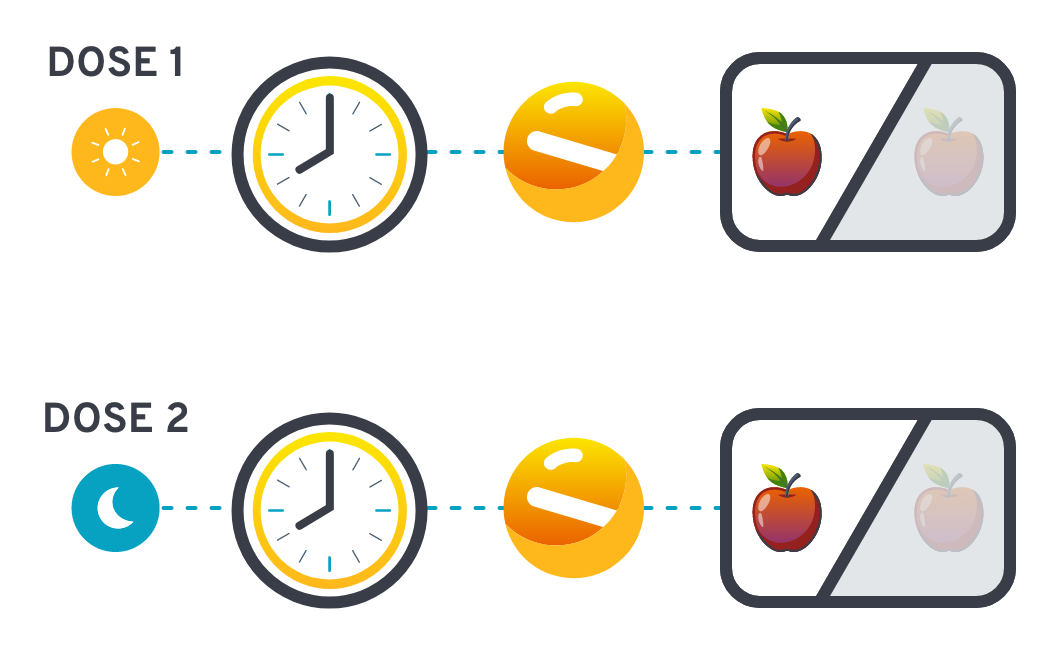
WAYRILZ can be taken at
approximately the same time each
day with or without food

WAYRILZ tablets should be swallowed whole with water and should not be cut, crushed, or chewed

Verify pregnancy status of females of reproductive potential prior to initiating WAYRILZ treatment
Monitoring and dose modifications for hepatotoxicity1
The value of WAYRILZ
Explore the value of WAYRILZ for your members with ITP.
References: 1. Wayrilz. Prescribing information. Sanofi Inc; 2025. 2. Langrish CL, Bradshaw JM, Francesco MR, et al. Preclinical efficacy and anti-inflammatory mechanisms of action of the Bruton tyrosine kinase inhibitor rilzabrutinib for immune-mediated disease. J Immunol. 2021;206(7):1454-1468. doi:10.4049/jimmunol.2001130 3. Kuter DJ, Bussel JB, Ghanima W, et al. Rilzabrutinib versus placebo in adults and adolescents with persistent or chronic immune thrombocytopenia: LUNA 3 phase III study. Ther Adv Hematol. 2023;14:20406207231205431. doi:10.1177/20406207231205431 4. Data on File. Sanofi; 2025. 5. Ghanima W et al. Abstract presented at: 33rd Congress of the International Society of Thrombosis and Haemostasis (ISTH); June 21-25,2025; Washington, DC. 6. Kuter DJ, Ghanima W, Cooper N, et al. LUNA3 open-label period: first efficacy/safety with rilzabrutinib in previously treated ITP adults. Poster presented at: 33rd Congress of the International Society of Thrombosis and Haemostasis (ISTH); June 21-25,2025; Washington, DC. 5. Data on File. Sanofi; 2025.
INDICATION
WAYRILZ is indicated for the treatment of adult patients with persistent or chronic immune thrombocytopenia (ITP) who have had an insufficient response to a previous treatment.
WARNINGS AND PRECAUTIONS
Serious Infections: An increased risk of serious infections (including bacterial, viral, or fungal) can occur in patients treated with Bruton’s tyrosine kinase (BTK) inhibitors, including WAYRILZ. Fatal pneumonia occurred in one participant treated with WAYRILZ in the LUNA-3 trial. Other serious infections [one each (0.8%)] included COVID-19 infection, wound infection, urinary tract infection and kidney abscess. Monitor patients for signs and symptoms of infection and treat appropriately.
Hepatotoxicity, Including Drug-Induced Liver Injury (DILI): Hepatotoxicity, including severe, life-threatening, and potentially fatal cases of DILI, can occur in patients treated with BTK inhibitors. Elevations of liver transaminases occurred with WAYRILZ in the ITP clinical trials and were generally mild to moderate in severity. Evaluate bilirubin and transaminases at baseline and as clinically indicated during treatment with WAYRILZ. For patients who develop abnormal liver tests after WAYRILZ, monitor more frequently. If DILI is suspected, withhold WAYRILZ. Upon confirmation of DILI, discontinue WAYRILZ.
Embryo-Fetal Toxicity: Based on findings from preliminary animal reproduction studies, WAYRILZ may cause fetal harm when administered to a pregnant woman. Verify pregnancy status of females of reproductive potential prior to initiating WAYRILZ treatment. Advise females of reproductive potential to use effective contraception while taking WAYRILZ and for 1 week after the final dose.
ADVERSE REACTIONS
Most common adverse reactions reported (incidence ≥10%) were diarrhea, nausea, headache, abdominal pain, and COVID-19.
DRUG INTERACTIONS
- Avoid concomitant use of WAYRILZ with strong or moderate CYP3A inhibitors, which increases the risk of WAYRILZ adverse reactions. If short term use of these inhibitors cannot be avoided, interrupt treatment with WAYRILZ. Avoid concomitant use of grapefruit, starfruit and products containing these fruits, and Seville oranges with WAYRILZ.
- Avoid concomitant use with a strong or moderate CYP3A inducer, which may reduce WAYRILZ efficacy.
- Administer the dose of WAYRILZ at least 2 hours before administration of an antacid or histamine H2 receptor antagonist. Avoid concomitant use of proton pump inhibitors with WAYRILZ. Concomitant use of acid reducing agents may reduce WAYRILZ efficacy.
- Rilzabrutinib is a moderate inhibitor of CYP3A and increases exposure of these substrates. Monitor for adverse reactions and consider dosage adjustment of the CYP3A substrate.
- Rilzabrutinib is an inhibitor of P-gp, BCRP and OATP1B in vitro. The effect of concomitant use of WAYRILZ with OATP1B and BCRP substrates has not been established in clinical studies. Monitor for adverse reactions of the concurrently administered P-gp, BCRP, or OATP1B substrate more frequently where minimal substrate concentration changes may lead to serious adverse reactions.
USE IN SPECIFIC POPULATIONS
- Lactation: Due to the potential for adverse reactions in a breastfed child, advise lactating women not to breastfeed while taking WAYRILZ and for at least 1 week after the final dose
- Hepatic Impairment: Avoid administration of WAYRILZ in patients with moderate or severe hepatic impairment (Child-Pugh class B-C)
- Renal Impairment: Avoid use in patients with severe renal impairment
Please see full Prescribing Information.
Learn more about Sanofi's commitment to fighting counterfeit drugs.
INDICATION
WAYRILZ is indicated for the treatment of adult patients with persistent or chronic immune thrombocytopenia (ITP) who have had an insufficient response to a previous treatment.
WARNINGS AND PRECAUTIONS
Serious Infections: An increased risk of serious infections (including bacterial, viral, or fungal) can occur in patients treated with Bruton’s tyrosine kinase (BTK) inhibitors, including WAYRILZ. Fatal pneumonia occurred in one participant treated with WAYRILZ in the LUNA-3 trial. Other serious infections [one each (0.8%)] included COVID-19 infection, wound infection, urinary tract infection and kidney abscess. Monitor patients for signs and symptoms of infection and treat appropriately.
Hepatotoxicity, Including Drug-Induced Liver Injury (DILI): Hepatotoxicity, including severe, life-threatening, and potentially fatal cases of DILI, can occur in patients treated with BTK inhibitors. Elevations of liver transaminases occurred with WAYRILZ in the ITP clinical trials and were generally mild to moderate in severity. Evaluate bilirubin and transaminases at baseline and as clinically indicated during treatment with WAYRILZ. For patients who develop abnormal liver tests after WAYRILZ, monitor more frequently. If DILI is suspected, withhold WAYRILZ. Upon confirmation of DILI, discontinue WAYRILZ.
Embryo-Fetal Toxicity: Based on findings from preliminary animal reproduction studies, WAYRILZ may cause fetal harm when administered to a pregnant woman. Verify pregnancy status of females of reproductive potential prior to initiating WAYRILZ treatment. Advise females of reproductive potential to use effective contraception while taking WAYRILZ and for 1 week after the final dose.
ADVERSE REACTIONS
Most common adverse reactions reported (incidence ≥10%) were diarrhea, nausea, headache, abdominal pain, and COVID-19.
DRUG INTERACTIONS
- Avoid concomitant use of WAYRILZ with strong or moderate CYP3A inhibitors, which increases the risk of WAYRILZ adverse reactions. If short term use of these inhibitors cannot be avoided, interrupt treatment with WAYRILZ. Avoid concomitant use of grapefruit, starfruit and products containing these fruits, and Seville oranges with WAYRILZ.
- Avoid concomitant use with a strong or moderate CYP3A inducer, which may reduce WAYRILZ efficacy.
- Administer the dose of WAYRILZ at least 2 hours before administration of an antacid or histamine H2 receptor antagonist. Avoid concomitant use of proton pump inhibitors with WAYRILZ. Concomitant use of acid reducing agents may reduce WAYRILZ efficacy.
- Rilzabrutinib is a moderate inhibitor of CYP3A and increases exposure of these substrates. Monitor for adverse reactions and consider dosage adjustment of the CYP3A substrate.
- Rilzabrutinib is an inhibitor of P-gp, BCRP and OATP1B in vitro. The effect of concomitant use of WAYRILZ with OATP1B and BCRP substrates has not been established in clinical studies. Monitor for adverse reactions of the concurrently administered P-gp, BCRP, or OATP1B substrate more frequently where minimal substrate concentration changes may lead to serious adverse reactions.
USE IN SPECIFIC POPULATIONS
- Lactation: Due to the potential for adverse reactions in a breastfed child, advise lactating women not to breastfeed while taking WAYRILZ and for at least 1 week after the final dose
- Hepatic Impairment: Avoid administration of WAYRILZ in patients with moderate or severe hepatic impairment (Child-Pugh class B-C)
- Renal Impairment: Avoid use in patients with severe renal impairment
Please see full Prescribing Information.
Learn more about Sanofi's commitment to fighting counterfeit drugs.
INDICATION
WAYRILZ is indicated for the treatment of adult patients with persistent or chronic immune thrombocytopenia (ITP) who have had an insufficient response to a previous treatment.
WARNINGS AND PRECAUTIONS
Serious Infections: An increased risk of serious infections (including bacterial, viral, or fungal) can occur in patients treated with Bruton’s tyrosine kinase (BTK) inhibitors, including WAYRILZ. Fatal pneumonia occurred in one participant treated with WAYRILZ in the LUNA-3 trial. Other serious infections [one each (0.8%)] included COVID-19 infection, wound infection, urinary tract infection and kidney abscess. Monitor patients for signs and symptoms of infection and treat appropriately.
Hepatotoxicity, Including Drug-Induced Liver Injury (DILI): Hepatotoxicity, including severe, life-threatening, and potentially fatal cases of DILI, can occur in patients treated with BTK inhibitors. Elevations of liver transaminases occurred with WAYRILZ in the ITP clinical trials and were generally mild to moderate in severity. Evaluate bilirubin and transaminases at baseline and as clinically indicated during treatment with WAYRILZ. For patients who develop abnormal liver tests after WAYRILZ, monitor more frequently. If DILI is suspected, withhold WAYRILZ. Upon confirmation of DILI, discontinue WAYRILZ.
Embryo-Fetal Toxicity: Based on findings from preliminary animal reproduction studies, WAYRILZ may cause fetal harm when administered to a pregnant woman. Verify pregnancy status of females of reproductive potential prior to initiating WAYRILZ treatment. Advise females of reproductive potential to use effective contraception while taking WAYRILZ and for 1 week after the final dose.
ADVERSE REACTIONS
Most common adverse reactions reported (incidence ≥10%) were diarrhea, nausea, headache, abdominal pain, and COVID-19.
DRUG INTERACTIONS
- Avoid concomitant use of WAYRILZ with strong or moderate CYP3A inhibitors, which increases the risk of WAYRILZ adverse reactions. If short term use of these inhibitors cannot be avoided, interrupt treatment with WAYRILZ. Avoid concomitant use of grapefruit, starfruit and products containing these fruits, and Seville oranges with WAYRILZ.
- Avoid concomitant use with a strong or moderate CYP3A inducer, which may reduce WAYRILZ efficacy.
- Administer the dose of WAYRILZ at least 2 hours before administration of an antacid or histamine H2 receptor antagonist. Avoid concomitant use of proton pump inhibitors with WAYRILZ. Concomitant use of acid reducing agents may reduce WAYRILZ efficacy.
- Rilzabrutinib is a moderate inhibitor of CYP3A and increases exposure of these substrates. Monitor for adverse reactions and consider dosage adjustment of the CYP3A substrate.
- Rilzabrutinib is an inhibitor of P-gp, BCRP and OATP1B in vitro. The effect of concomitant use of WAYRILZ with OATP1B and BCRP substrates has not been established in clinical studies. Monitor for adverse reactions of the concurrently administered P-gp, BCRP, or OATP1B substrate more frequently where minimal substrate concentration changes may lead to serious adverse reactions.
USE IN SPECIFIC POPULATIONS
- Lactation: Due to the potential for adverse reactions in a breastfed child, advise lactating women not to breastfeed while taking WAYRILZ and for at least 1 week after the final dose
- Hepatic Impairment: Avoid administration of WAYRILZ in patients with moderate or severe hepatic impairment (Child-Pugh class B-C)
- Renal Impairment: Avoid use in patients with severe renal impairment
Please see full Prescribing Information.
Learn more about Sanofi's commitment to fighting counterfeit drugs.
© 2025 Sanofi. All rights reserved.
WAYRILZ, HemAssist, and Sanofi are trademarks of Sanofi or an affiliate.
All other trademarks above are the property of their respective owners, who have no affiliation or relationship with Sanofi.