Treatment landscape:
ITP overview and unmet treatment needs
Immune thrombocytopenia (ITP) overview
ITP is a rare, potentially life-threatening immune dysregulation disorder impacting approximately 170,000 patients in the United States1-5
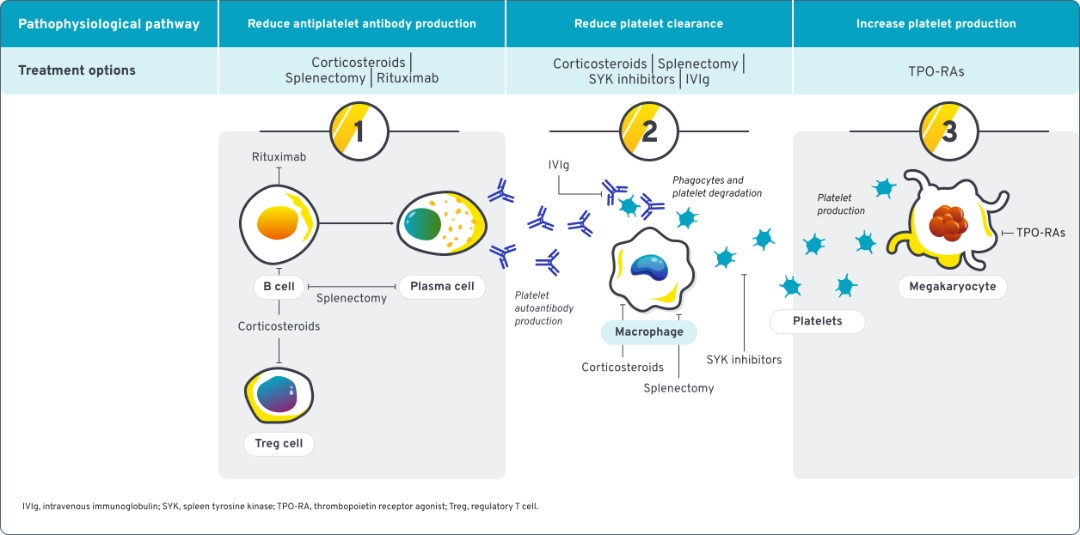
ITP is a disease of complex immune dysregulation that involves multiple components of the immune system driving platelet destruction.5,6
ITP is a diagnosis of exclusion with 3 distinct disease phases after diagnosis9
ITP symptoms and symptom severity vary from patient to patient1,4,10,11
|
|
|
|---|---|
| 150–450 × 10⁹/L | Normal platelet count in adults |
| <100 × 10⁹/L | Platelet count when ITP is often diagnosed |
| <20 × 10⁹/L | Platelet count when ITP is clinically treated |
| <10 × 10⁹/L | Risk of severe bleeding, including intracranial and other internal bleeding |

ITP is associated with increased bleeding risk and thromboembolic risk

Bleeding occurs in ~60% of patients; severe bleeding occurs in ~7% of patients14,15,a

Patients aged >60 years with ITP who experience intracerebral hemorrhage have a 50% to 80% mortality rate, making it the most common ITP-related cause of death12
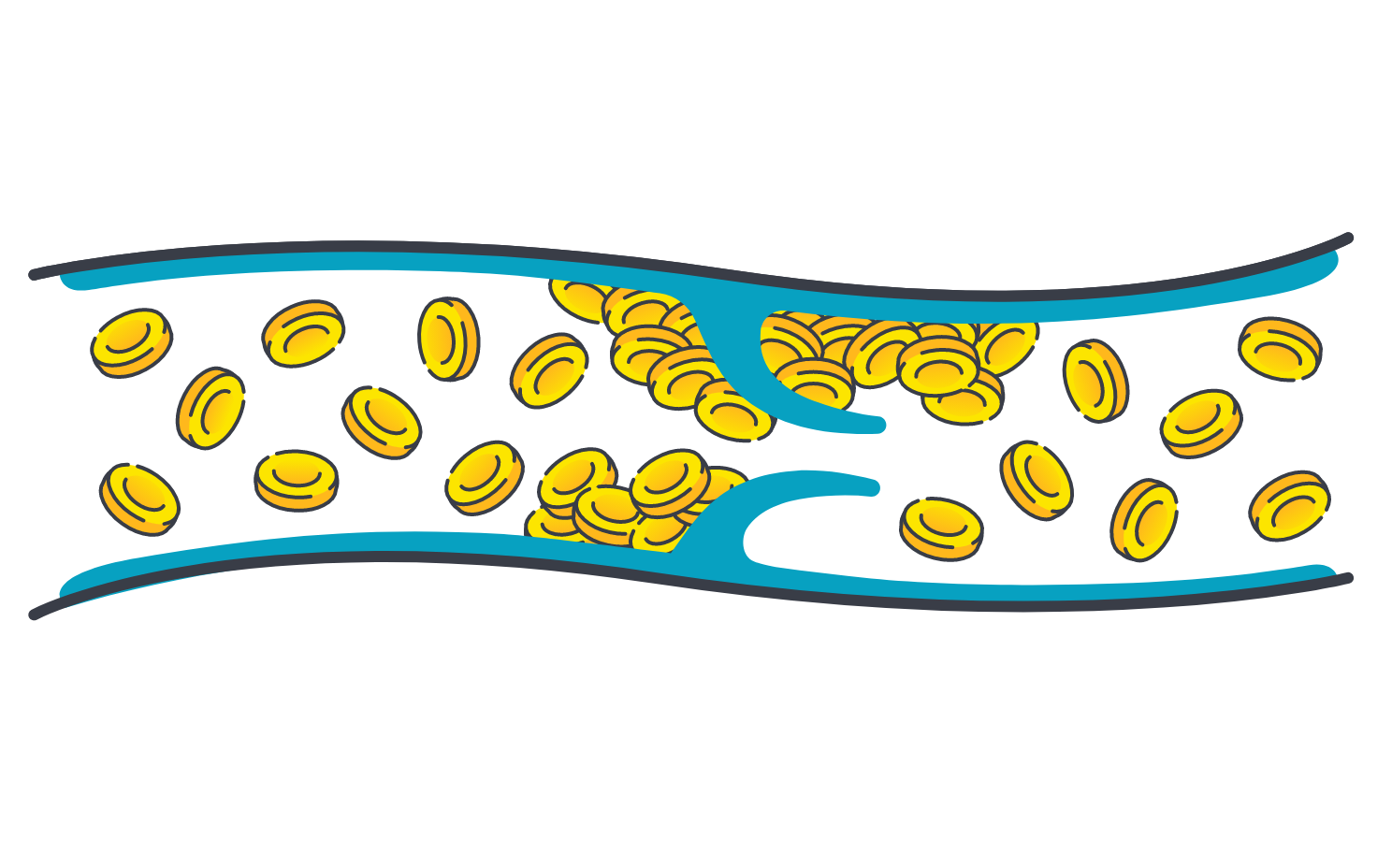
Thrombosis has been reported in up to 8% of patients with ITP16
Reduced quality of life is a commonly reported symptom of ITP19,a
Unmet treatment needs
Current treatments for ITP may not address all aspects of complex immune dysregulation20
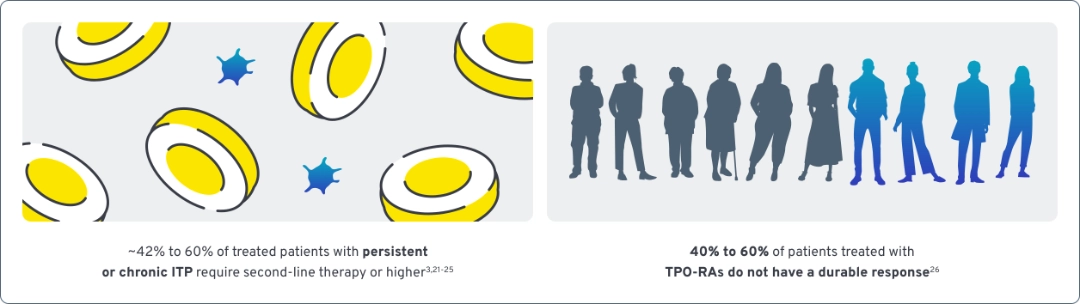
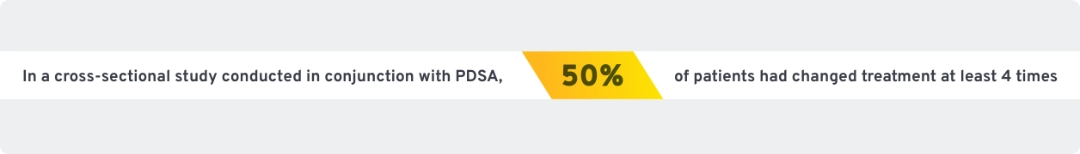
Many patients with ITP cycle through treatment options as unmet needs persist
Reasons cited for switching were:
Insufficient platelet response
Reduced efficacy over time
Symptoms and/or side effects
Many patients with ITP cycle through treatment options in search of results29
Discover WAYRILZ
Take a closer look at the mechanism of action and clinical results for WAYRILZ.
References: 1. Vrbensky JR, Moore JE, Arnold DM, Smith JW, Kelton JG, Nazy I. The sensitivity and specificity of platelet autoantibody testing in immune thrombocytopenia: a systematic review and meta-analysis of a diagnostic test. J Thromb Haemost. 2019;17(5):787-794. doi:10.1111/jth.14419 2. Age and sex composition in the United States: 2021. US Census Bureau. October 2021. Accessed August 25, 2025. https://www.census.gov/data/tables/2021/demo/age-and-sex/2021-age-sex-composition.html 3. Data on file. Optum database, 2023 analysis of 2021 population. Sanofi US Inc. 2025. 4. Doobaree IU, Conway K, Miah H, et al. Incidence of adult primary immune thrombocytopenia in England—an update. Eur J Haematol. 2022;109(3):238-249. doi:10.1111/ejh.13803 5. Cooper N, Ghanima W. Immune thrombocytopenia. N Engl J Med. 2019;381(10):945-955. doi:10.1056/NEJMcp1810479 6. Hill QA, Newland AC. Fatigue in immune thrombocytopenia. Br J Haematol. 2015;170(2):141-149. doi:10.1111/bjh.13385 7. Kashiwagi H, Tomiyama Y. Pathophysiology and management of primary immune thrombocytopenia. Int J Hematol. 2013;98(1):24-33. doi:10.1007/s12185-013-1370-4 8. Wang M, Chen Z, Wong M, et al. Are the correct outcomes being measured in studies of oral anticoagulants? A systematic survey. Thromb Res. 2021;201:30-49. doi:10.1016/j.thromres.2021.02.016 9. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393. doi:10.1182/blood-2008-07-162503 10. Schoonen WM, Kucera G, Coalson J, et al. Epidemiology of immune thrombocytopenic purpura in the General Practice Research Database [published correction appears in Br J Haematol. 2009;147(1):157]. Br J Haematol. 2009;145(2):235-244. doi:10.1111/j.1365-2141.2009.07615.x 11. Zufferey A, Kapur R, Semple JW. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP). J Clin Med. 2017;6(2):16. doi:10.3390/jcm6020016 12. Al-Samkari H, Kuter DJ. Immune thrombocytopenia in adults: modern approaches to diagnosis and treatment. Semin Thromb Hemost. 2020;46(3):275-288. doi:10.1055/s-0039-1700512 13. Vladescu C, Hart ACJ, Paul D, et al. Cognitive impairment in patients with immune thrombocytopenia. Blood. 2022;140(suppl 1):5553-5554. doi:10.1182/blood-2022-166412 14. Piel-Julian ML, Mahévas M, Germain J, et al. Risk factors for bleeding, including platelet count threshold, in newly diagnosed immune thrombocytopenia adults. J Thromb Haemost. 2018;16(9):1830-1842. doi:10.1111/jth.14227 15. Audia S, Bonnotte B. Emerging therapies in immune thrombocytopenia. J Clin Med. 2021;10(5):1004. doi:10.3390/jcm10051004 16. Takagi S, Suzuki I, Watanabe S. Risk of thromboembolism in patients with immune thrombocytopenia. J Hematol Thrombo Dis. 2015;3(1):1000185. doi:10.3324/haematol.2018.212845 17. Swan D, Newland A, Rodeghiero F, Thachil J. Thrombosis in immune thrombocytopenia – current status and future perspectives. Br J Haematol. 2021;194(5):822-834. doi:10.1111/bjh.17390 18. Langeberg WJ, Schoonen WM, Eisen M, Gamelin L, Stryker S. Thromboembolism in patients with immune thrombocytopenia (ITP): a meta-analysis of observational studies. Int J Hematol. 2016;103(6):655-664. doi:10.1007/s12185-016-1974-6 19. Cooper N, Kruse A, Kruse C, et al. Immune thrombocytopenia (ITP) World Impact Survey (iWISh): patient and physician perceptions of diagnosis, signs and symptoms, and treatment. Am J Hematol. 2021;96(2):188-198. doi:10.1002/ajh.26045 20. Singh A, Uzun G, Bakchoul T. Primary immune thrombocytopenia: novel insights into pathophysiology and disease management. J Clin Med. 2021;10(4):789. doi:10.3390/jcm10040789 21. Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829-3866. doi:10.1182/bloodadvances.2019000966 22. González-López TJ, Schifferli A. Early immunomodulation in immune thrombocytopenia—a report of the ICIS meeting in Lenzerheide, Switzerland 2022. Br J Haematol. 2023;203(1):101-111. doi:10.1111/bjh.19082 23. Witkowski M, Witkowska M, Robak T. Autoimmune thrombocytopenia: current treatment options in adults with a focus on novel drugs. Eur J Haematol. 2019;103(6):531-541. doi:10.1111/ejh.13319 24. Wei Y, Ji XB, Wang YW, et al. High-dose dexamethasone vs prednisone for treatment of adult immune thrombocytopenia: a prospective multicenter randomized trial. Blood. 2016;127(3):296-302. doi:10.1182/blood-2015-07-659656 25. Data on file. ITP – rilzabrutinib demand assessment. US 2023. Sanofi US Inc. 2025. 26. Ghanima W, Cooper N, Rodeghiero F, Godeau B, Bussel JB. Thrombopoietin receptor agonists: ten years later. Haematologica. 2019;104(6):1112-1123. doi:10.3324/haematol.2018.212845 27. Mingot-Castellano ME, Bastida JM, Caballero-Navarro G, et al. Novel therapies to address unmet needs in ITP. Pharmaceuticals (Basel). 2022;15(7):779. doi:10.3390/ph15070779 28. Promacta. Prescribing information. Novartis; 2025. 29. Cooper N, Kruse C, Morgan SD, et al. Identifying unmet needs related to treatment and disease control in immune thrombocytopenia (ITP): US patient survey. Poster presented at: 32nd Congress of the International Society on Thrombosis and Haemostasis (ISTH); June 22-26, 2024; Bangkok, Thailand.
INDICATION
WAYRILZ is indicated for the treatment of adult patients with persistent or chronic immune thrombocytopenia (ITP) who have had an insufficient response to a previous treatment.
WARNINGS AND PRECAUTIONS
Serious Infections: An increased risk of serious infections (including bacterial, viral, or fungal) can occur in patients treated with Bruton’s tyrosine kinase (BTK) inhibitors, including WAYRILZ. Fatal pneumonia occurred in one participant treated with WAYRILZ in the LUNA-3 trial. Other serious infections [one each (0.8%)] included COVID-19 infection, wound infection, urinary tract infection and kidney abscess. Monitor patients for signs and symptoms of infection and treat appropriately.
Hepatotoxicity, Including Drug-Induced Liver Injury (DILI): Hepatotoxicity, including severe, life-threatening, and potentially fatal cases of DILI, can occur in patients treated with BTK inhibitors. Elevations of liver transaminases occurred with WAYRILZ in the ITP clinical trials and were generally mild to moderate in severity. Evaluate bilirubin and transaminases at baseline and as clinically indicated during treatment with WAYRILZ. For patients who develop abnormal liver tests after WAYRILZ, monitor more frequently. If DILI is suspected, withhold WAYRILZ. Upon confirmation of DILI, discontinue WAYRILZ.
Embryo-Fetal Toxicity: Based on findings from preliminary animal reproduction studies, WAYRILZ may cause fetal harm when administered to a pregnant woman. Verify pregnancy status of females of reproductive potential prior to initiating WAYRILZ treatment. Advise females of reproductive potential to use effective contraception while taking WAYRILZ and for 1 week after the final dose.
ADVERSE REACTIONS
Most common adverse reactions reported (incidence ≥10%) were diarrhea, nausea, headache, abdominal pain, and COVID-19.
DRUG INTERACTIONS
- Avoid concomitant use of WAYRILZ with strong or moderate CYP3A inhibitors, which increases the risk of WAYRILZ adverse reactions. If short term use of these inhibitors cannot be avoided, interrupt treatment with WAYRILZ. Avoid concomitant use of grapefruit, starfruit and products containing these fruits, and Seville oranges with WAYRILZ.
- Avoid concomitant use with a strong or moderate CYP3A inducer, which may reduce WAYRILZ efficacy.
- Administer the dose of WAYRILZ at least 2 hours before administration of an antacid or histamine H2 receptor antagonist. Avoid concomitant use of proton pump inhibitors with WAYRILZ. Concomitant use of acid reducing agents may reduce WAYRILZ efficacy.
- Rilzabrutinib is a moderate inhibitor of CYP3A and increases exposure of these substrates. Monitor for adverse reactions and consider dosage adjustment of the CYP3A substrate.
- Rilzabrutinib is an inhibitor of P-gp, BCRP and OATP1B in vitro. The effect of concomitant use of WAYRILZ with OATP1B and BCRP substrates has not been established in clinical studies. Monitor for adverse reactions of the concurrently administered P-gp, BCRP, or OATP1B substrate more frequently where minimal substrate concentration changes may lead to serious adverse reactions.
USE IN SPECIFIC POPULATIONS
- Lactation: Due to the potential for adverse reactions in a breastfed child, advise lactating women not to breastfeed while taking WAYRILZ and for at least 1 week after the final dose
- Hepatic Impairment: Avoid administration of WAYRILZ in patients with moderate or severe hepatic impairment (Child-Pugh class B-C)
- Renal Impairment: Avoid use in patients with severe renal impairment
Please see full Prescribing Information.
Learn more about Sanofi's commitment to fighting counterfeit drugs.
INDICATION
WAYRILZ is indicated for the treatment of adult patients with persistent or chronic immune thrombocytopenia (ITP) who have had an insufficient response to a previous treatment.
WARNINGS AND PRECAUTIONS
Serious Infections: An increased risk of serious infections (including bacterial, viral, or fungal) can occur in patients treated with Bruton’s tyrosine kinase (BTK) inhibitors, including WAYRILZ. Fatal pneumonia occurred in one participant treated with WAYRILZ in the LUNA-3 trial. Other serious infections [one each (0.8%)] included COVID-19 infection, wound infection, urinary tract infection and kidney abscess. Monitor patients for signs and symptoms of infection and treat appropriately.
Hepatotoxicity, Including Drug-Induced Liver Injury (DILI): Hepatotoxicity, including severe, life-threatening, and potentially fatal cases of DILI, can occur in patients treated with BTK inhibitors. Elevations of liver transaminases occurred with WAYRILZ in the ITP clinical trials and were generally mild to moderate in severity. Evaluate bilirubin and transaminases at baseline and as clinically indicated during treatment with WAYRILZ. For patients who develop abnormal liver tests after WAYRILZ, monitor more frequently. If DILI is suspected, withhold WAYRILZ. Upon confirmation of DILI, discontinue WAYRILZ.
Embryo-Fetal Toxicity: Based on findings from preliminary animal reproduction studies, WAYRILZ may cause fetal harm when administered to a pregnant woman. Verify pregnancy status of females of reproductive potential prior to initiating WAYRILZ treatment. Advise females of reproductive potential to use effective contraception while taking WAYRILZ and for 1 week after the final dose.
ADVERSE REACTIONS
Most common adverse reactions reported (incidence ≥10%) were diarrhea, nausea, headache, abdominal pain, and COVID-19.
DRUG INTERACTIONS
- Avoid concomitant use of WAYRILZ with strong or moderate CYP3A inhibitors, which increases the risk of WAYRILZ adverse reactions. If short term use of these inhibitors cannot be avoided, interrupt treatment with WAYRILZ. Avoid concomitant use of grapefruit, starfruit and products containing these fruits, and Seville oranges with WAYRILZ.
- Avoid concomitant use with a strong or moderate CYP3A inducer, which may reduce WAYRILZ efficacy.
- Administer the dose of WAYRILZ at least 2 hours before administration of an antacid or histamine H2 receptor antagonist. Avoid concomitant use of proton pump inhibitors with WAYRILZ. Concomitant use of acid reducing agents may reduce WAYRILZ efficacy.
- Rilzabrutinib is a moderate inhibitor of CYP3A and increases exposure of these substrates. Monitor for adverse reactions and consider dosage adjustment of the CYP3A substrate.
- Rilzabrutinib is an inhibitor of P-gp, BCRP and OATP1B in vitro. The effect of concomitant use of WAYRILZ with OATP1B and BCRP substrates has not been established in clinical studies. Monitor for adverse reactions of the concurrently administered P-gp, BCRP, or OATP1B substrate more frequently where minimal substrate concentration changes may lead to serious adverse reactions.
USE IN SPECIFIC POPULATIONS
- Lactation: Due to the potential for adverse reactions in a breastfed child, advise lactating women not to breastfeed while taking WAYRILZ and for at least 1 week after the final dose
- Hepatic Impairment: Avoid administration of WAYRILZ in patients with moderate or severe hepatic impairment (Child-Pugh class B-C)
- Renal Impairment: Avoid use in patients with severe renal impairment
Please see full Prescribing Information.
Learn more about Sanofi's commitment to fighting counterfeit drugs.
INDICATION
WAYRILZ is indicated for the treatment of adult patients with persistent or chronic immune thrombocytopenia (ITP) who have had an insufficient response to a previous treatment.
WARNINGS AND PRECAUTIONS
Serious Infections: An increased risk of serious infections (including bacterial, viral, or fungal) can occur in patients treated with Bruton’s tyrosine kinase (BTK) inhibitors, including WAYRILZ. Fatal pneumonia occurred in one participant treated with WAYRILZ in the LUNA-3 trial. Other serious infections [one each (0.8%)] included COVID-19 infection, wound infection, urinary tract infection and kidney abscess. Monitor patients for signs and symptoms of infection and treat appropriately.
Hepatotoxicity, Including Drug-Induced Liver Injury (DILI): Hepatotoxicity, including severe, life-threatening, and potentially fatal cases of DILI, can occur in patients treated with BTK inhibitors. Elevations of liver transaminases occurred with WAYRILZ in the ITP clinical trials and were generally mild to moderate in severity. Evaluate bilirubin and transaminases at baseline and as clinically indicated during treatment with WAYRILZ. For patients who develop abnormal liver tests after WAYRILZ, monitor more frequently. If DILI is suspected, withhold WAYRILZ. Upon confirmation of DILI, discontinue WAYRILZ.
Embryo-Fetal Toxicity: Based on findings from preliminary animal reproduction studies, WAYRILZ may cause fetal harm when administered to a pregnant woman. Verify pregnancy status of females of reproductive potential prior to initiating WAYRILZ treatment. Advise females of reproductive potential to use effective contraception while taking WAYRILZ and for 1 week after the final dose.
ADVERSE REACTIONS
Most common adverse reactions reported (incidence ≥10%) were diarrhea, nausea, headache, abdominal pain, and COVID-19.
DRUG INTERACTIONS
- Avoid concomitant use of WAYRILZ with strong or moderate CYP3A inhibitors, which increases the risk of WAYRILZ adverse reactions. If short term use of these inhibitors cannot be avoided, interrupt treatment with WAYRILZ. Avoid concomitant use of grapefruit, starfruit and products containing these fruits, and Seville oranges with WAYRILZ.
- Avoid concomitant use with a strong or moderate CYP3A inducer, which may reduce WAYRILZ efficacy.
- Administer the dose of WAYRILZ at least 2 hours before administration of an antacid or histamine H2 receptor antagonist. Avoid concomitant use of proton pump inhibitors with WAYRILZ. Concomitant use of acid reducing agents may reduce WAYRILZ efficacy.
- Rilzabrutinib is a moderate inhibitor of CYP3A and increases exposure of these substrates. Monitor for adverse reactions and consider dosage adjustment of the CYP3A substrate.
- Rilzabrutinib is an inhibitor of P-gp, BCRP and OATP1B in vitro. The effect of concomitant use of WAYRILZ with OATP1B and BCRP substrates has not been established in clinical studies. Monitor for adverse reactions of the concurrently administered P-gp, BCRP, or OATP1B substrate more frequently where minimal substrate concentration changes may lead to serious adverse reactions.
USE IN SPECIFIC POPULATIONS
- Lactation: Due to the potential for adverse reactions in a breastfed child, advise lactating women not to breastfeed while taking WAYRILZ and for at least 1 week after the final dose
- Hepatic Impairment: Avoid administration of WAYRILZ in patients with moderate or severe hepatic impairment (Child-Pugh class B-C)
- Renal Impairment: Avoid use in patients with severe renal impairment
Please see full Prescribing Information.
Learn more about Sanofi's commitment to fighting counterfeit drugs.
© 2025 Sanofi. All rights reserved.
WAYRILZ, HemAssist, and Sanofi are trademarks of Sanofi or an affiliate.
All other trademarks above are the property of their respective owners, who have no affiliation or relationship with Sanofi.